
��15�֣���1��3.01��1022��OH¯�����ʵ���Ϊ ����ЩOH¯�� molNH3��������ȣ��� �� g Na+���е���������ͬ��
��2���ڱ�״���£�2.24LNOx���������Ϊ4.6g����x��ֵΪ ��
��3�����������εĻ����Һ������0.2 mol��L��1 Na+��0.25 mol��L��1 Mg2+��0.4mol��L��1
Cl������SO ��Ũ��Ϊ�� ��
��Ũ��Ϊ�� ��
��4������14.2g���������Ƴ�500mL��Һ�������ʵ���Ũ��Ϊ mol/L��������ȡ��50mL�������ʵ���Ũ��Ϊ mol/L����Һ�к�Na+�ĸ���Ϊ
������50mL��Һ��ˮϡ�͵�100mL�� SO42-�����ʵ���Ũ��Ϊ mol/L��
��1��0.05�� 0.05��1.15 ��ÿ��1�֣�
��2��2 ��2�֣� ��3��0.15mol/L ��2�֣�
(4) 0.2 mol/L�� 0.02NA��0.2mol/L��0.1 mol/L����ÿ��2�֣�
����������1��3.01��1022��OH¯���ӵ����ʵ�����3.01��1022��6.02��1023/mol��0.05mol��������Ħ��������OH¯��Ħ��������ͬ�������������ʵ�����0.05mol�������ӵ����ʵ�����0.05mol��������0.05mol��23g/mol��1.15g��
��2����״���£�2.24LNOx��������ʵ�����0.1mol������������Է���������46������x��2��
��3����Һ�ǵ����Եģ�����SO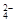 ��Ũ��Ϊ��0.2
mol��L��1 ��2��0.25 mol��L��1 ��0.4mol��L��1����2��0.15mol/L��
��Ũ��Ϊ��0.2
mol��L��1 ��2��0.25 mol��L��1 ��0.4mol��L��1����2��0.15mol/L��
��4��14.2g�����Ƶ����ʵ�����14.2g��142g/mol��0.1mol�������c��n/V��֪��Ũ����0.1mol��0.5L��0.2 mol/L����Һ�Ǿ�һ�ģ����Ũ�Ȳ��䣻��Һ�к�Na+�ĸ���Ϊ0.05L��0.4mol/L��NA��0.02NA����ϡ�����У������Dz���ģ�����ϡ�ͺ��Ũ����0.2mol/L��2��0.1 mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ���������и߶���ѧ�����п��Ի�ѧ���ģ��Ծ� ���ͣ���ѡ��
����˵����ȷ����
| A��0.25 mol CO2 | B��1 mol����6.02��10 23������ |
| C��1 mol �� | D��0.5 mol H2 ���� 3.01��10 23 ����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016���Ĵ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��1��3.01��1022��OH�� �����ʵ���Ϊ________������Ϊ________���������ӵ����ʵ���Ϊ________�����е��ӵ����ʵ���Ϊ________��
��2����ͬ״���£�10mL X2������30mL Y2���廯������20mL C���壬��C�Ļ�ѧʽΪ_________��
��3��ij�����꾭���飬�����������⣬�����������ӣ�c(Na+)=1.4��10-3 mol��L-1 ��
c(Cl�� ) =3.5��10-3 mol��L-1 ��c(NH4+) =2.3��10-3 mol��L-1 �� c(SO42-) =1.5��10-3
mol��L-1�������Һ�������ӵ�Ũ��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и߶���ѧ�����п��Ի�ѧ���ģ��Ծ� ���ͣ�ѡ����
����˵����ȷ����
A. 0.25 mol CO2 B. 1 mol����6.02��10 23������
C. 1 mol �� D. 0.5 mol H2 ���� 3.01��10 23 ����ԭ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com