
| A�� | c��NH4+����ȵ�NH4HCO3��Һ����NH4��2Fe��SO4��2��Һ�ͣ�NH4��2SO4��Һ�У�����Ũ�ȴ�С��ϵ�ǣ�c[��NH4��2Fe��SO4��2]��c[��NH4��2SO4]��c��NH4HCO3�� | |
| B�� | �����£�0.1 mol•L-1HCl��Һ������0.1 mol•L-1Ba��OH��2��Һ��Ϻ���Һ��pH=13 | |
| C�� | �����£���ˮ���������c��H+��=10-13 mol•L-1����Һ��Fe2+��NO3-��Cl-��SO42-һ�����ܴ������� | |
| D�� | 0.1 mol•L-1��NaHCO3��Һ�У�c��H+��+c��H2CO3��=c��CO32-��+c��OH-�� |
���� A��c��NH4+����ȣ���NH4��2Fe��SO4��2��Һ�ͣ�NH4��2SO4��Һ�к���2��NH4+��Fe2+����NH4+��ˮ�⣬Ũ�Ⱦ�С��NH4HCO3��Һ��
B�������£�����Һ���Ϊ1L��0.1mol��L-1��HCl��Һn��H+��=1L��0.1mol/L=0.1mol��1L0.1mol��L-1��Ba��OH��2��Һn��OH-��=2��1L��0.1mol/L=0.2mol�����������Ӧ����Һ�ʼ��ԣ����㷴Ӧ��c��OH-������ϸ��¶���Kw�ɼ���pH��
C�������£���ˮ���������c��H+��=10-13 mol•L-1����Һ����������Һ�����Һ���ݴ˷������ӹ��棻
D.0.1 mol•L-1��NaHCO3��Һ�д��ڵ���غ�������غ㣬�ݴ˼�������ж�����Ũ�ȴ�С��
��� �⣺A����NH4��2SO4 ����NH4��2Fe��SO4��2�к���2��NH4+����NH4��2SO4 ����NH4��2Fe��SO4��2��Ũ�Ⱦ�С��NH4HCO3��Һ����ΪFe2+����NH4+��ˮ�⣬����c[��NH4��2SO4 ]��c[��NH4��2Fe��SO4��2 ]����õ�����Ũ��c��NH4HCO3����c[��NH4��2SO4 ]��c[��NH4��2Fe��SO4��2 ]����A��ȷ��
B�������£�����Һ�����Ϊ1L��0.1mol��L-1��HCl��Һn��H+��=1L��0.1mol/L=0.1mol��1L0.1mol��L-1��Ba��OH��2��Һn��OH-��=2��1L��0.1mol/L=0.2mol�����������Һ�������Ϊ2L����Ӧ��ʣ������������Ũ��c��OH-��=$\frac{0.2mol-0.1mol}{2L}$=0.05mol/L��c��H+��=$\frac{1{0}^{-14}}{0.05}$=2��10-13mol/L��PH=13-lg2����B����
C�������£���ˮ���������c��H+��=10-13 mol•L-1����Һ����������Һ�����Һ������Һ��Fe2+��NO3-����������ԭ��Ӧ�����ܹ��棬����Һ��Fe2+���ܹ��棬��C��ȷ��
D.0.1 mol•L-1��NaHCO3��Һ�е���غ�c��Na+��+c��H+��=c��OH-��+c��HCO3-��+c��CO32-���������غ�c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3�����������õ�c��H+��+c��H2CO3��=c��CO32-��+c��OH-������D��ȷ��
��ѡB��
���� ���⿼��������ˮ�⡢�������Һ������Ũ�ȴ�С�Ƚϡ�PH���㡢����غ�������غ�ļ���Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
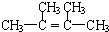 ��
�� ��
��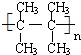 �ڴ��������£���H2�ӳɺ�IJ�������Ϊ2��3-�������飮
�ڴ��������£���H2�ӳɺ�IJ�������Ϊ2��3-�������飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 60g�����д��ڵĹ��ۼ�����Ϊ10NA | |
| B�� | 1L 0.1mol/L��NaHCO3��Һ��HCO3-��CO32-������֮��Ϊ0.1NA | |
| C�� | ���ڿ�����ȼ�տ����ɶ��������23 g�Ƴ��ȼ��ʱת�Ƶ�����Ϊ1NA | |
| D�� | �ܱ�������2mol NO��1molO2��ַ�Ӧ������ķ�����Ϊ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu$\stackrel{O_{2}��CO_{2}��H_{2}O}{��}$Cu2��OH��2CO3$\stackrel{��}{��}$Cu��OH��2 | |
| B�� | MgO$\stackrel{HCl��aq��}{��}$MgCl2��aq��$\stackrel{���ȣ�HCl��Χ}{��}$MgCl2��s�� | |
| C�� | CaCl2��aq��$\stackrel{CO_{2}}{��}$CaCO3$\stackrel{SiO_{2}/����}{��}$CaSiO3 | |
| D�� | S$\stackrel{O_{2}/��ȼ}{��}$SO3$\stackrel{H_{2}O}{��}$H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.4g����þ��Ϊþ����ʱʧȥ�ĵ�����Ϊ0.1NA | |
| B�� | 1molHCl�������������0.5mol/L������������������� | |
| C�� | 22.4L��CO������1molN2�����ĵ�������� | |
| D�� | �κ������£�16gCH4��18gH2O�����е�������Ϊ10NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | SO3+H2O�TH2SO4 | B�� | 2Na2O2+2H2O�T4NaOH+O2�� | ||
| C�� | 2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2�� | D�� | 3NO2+H2O�T2HNO3+NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
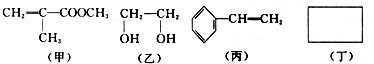
 ��-R������
��-R������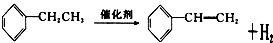
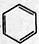 +CH3CH2Cl
+CH3CH2Cl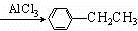 +HCl��
+HCl��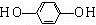 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ü�ȩ��Һ���ݺ���Ʒ���� | |
| B�� | ��ˮ�Ҵ�������ҽ�������� | |
| C�� | �˵�Ƥ����ǿ�����ߵ������½���ʧȥ�������� | |
| D�� | ���·������������ۻ�Ϊͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl��HCl��H2O��NaOH | B�� | Cl2��Na2S��HCl��CO2 | ||
| C�� | HBr��CCl4��H2O��CO2 | D�� | Na2O2��H2O2��H2O��O2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com