
�������ж�����Ԫ�����ʵ�������ݣ�
| �� | �� | �� | �� | �� | �� | |
| ԭ�Ӱ뾶��10��10m�� | 1.30 | 0.82 | 0.99 | 1.11 | 0.90 | 1.18 |
| �����ͻ��ϼ� | +2 | +3 | +7 | +4 | +2 | +3 |
| ��1 | ��4 |
��ش��������⣺
��1�� �ں͢��γɵĻ���������Ϊ ������ӡ����ۡ��������
��2��Ԫ�آٵĽ����Ա�Ԫ�آ�Ҫ ���ǿ�������������Դ�ԭ�ӽṹ���������ԭ�� ��
��3������Ȼ���У�Ԫ�آܵĴ�����̬Ϊ ����ҵ�ϴ��Ƹ�Ԫ�ص��ʵĻ�ѧ����ʽΪ ��
��4�� �ĵ�����ŨNaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��5��ʵ��������Ԫ�آٵĵ��ʺ�ijδ֪��������M�����Ҫд���Ƚ����߽�����ǿ����һ��ʵ�鷽�� ��
��1�����ۣ�2�֣�
��2��ǿ��1�֣���þ��ԭ�Ӱ뾶��������1�֣���ԭ�Ӻ˶��������ӵ�������������������ԭ�Ӹ�����ʧȥ���ӣ�1�֣�������þԪ�صĽ����Ա���Ҫǿ��
��3������̬��1�֣���![]() ��2�֣�
��2�֣�
��4��2Al �� 2OH�� �� 2H2O = 2AlO2�� �� 3H2�� ��2�֣�
��5��������С�ձ��зֱ������������ˮ�����Ũ�ȵ����ᣩ��1�֣���Ȼ��Ͷ����״��С��ͬ�Ľ���Ƭ��1�֣�����M��Ӧ��þ���ң���M�Ľ����Ա�Mgǿ ��1�֣�


 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ж�����Ԫ�����ʵ����ݣ�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� | a | b | c | d | e | f | g | h | i | j |
| ԭ�Ӱ뾶/pm | 111 | 77 | 70 | 104 | 143 | 99 | 117 | 186 | 160 | 64 |
| ����ϼۻ� ��ͻ��ϼ� |
+2 | -4 | -3 | +6 | +3 | -1 | +4 | +1 | +2 | -1 |
| ���� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | ||||||||
| 2 | a a |
b b |
c c |
j j |
||||
| 3 | h h |
i i |
e e |
g g |
d d |
f f |
||
| ||
| ||
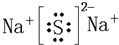
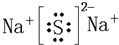


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Ԫ�ر�� Ԫ������ |
�� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 |
| ��������ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | |
| ��ͻ��ϼ� | -2 | -3 | -1 | -3 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر��Ԫ������ | �� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� Ԫ������ |
�� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶 ��10-10m�� |
0.74 | 1.02 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +6 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -2 | -3 | -1 | -3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com