
Ŀǰ���������һ�����Ա�����Ʒ������Ҫ�ɷ���ƻ���ᡣƻ�����ڷ����ᴿ��Ļ�ѧ�������£�����Է�������������250����ȫȼ�պ�ֻ����CO2��H2O��������C������������35.82%��H����������4.48%����1 mol������������NaHCO3��Ӧ�ų�44.8 L CO2����������Na��Ӧ�ų�33.6 L H2(����������ڱ�״���²���)���۸÷����д������ֻ�ѧ������ͬ��̼ԭ�ӣ���ԭ��Ҳ�������ֲ�ͬ�Ļ�ѧ�����С�
(1)д��ƻ����Ľṹ��ʽ��___________________________��
(2)��ƻ�����ͬ���칹���У����������٢�������������(д���ṹ��ʽ)��______________��
(3)д��ƻ����+�Ҵ�+������ŨH2SO4�����µĻ�ѧ����ʽ��___________________________��
(4)ƻ������Է�������ת����

��֪BΪ��Ԫ��״�ṹ��C��ʹ��ˮ��ɫ��E��������嵰���ʵİ�����֮һ�������ʽΪC4H7O4N��
��д����C������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ_________��E�Ľṹ��ʽ_________��
��D![]() E�ķ�Ӧ������_________��
E�ķ�Ӧ������_________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)д��ƻ����Ľṹ��ʽ��___________________________��
(2)��ƻ�����ͬ���칹���У����������٢�������������(д���ṹ��ʽ)��______________��
(3)д��ƻ����+�Ҵ�+������ŨH2SO4�����µĻ�ѧ����ʽ��___________________________��
(4)ƻ������Է�������ת����
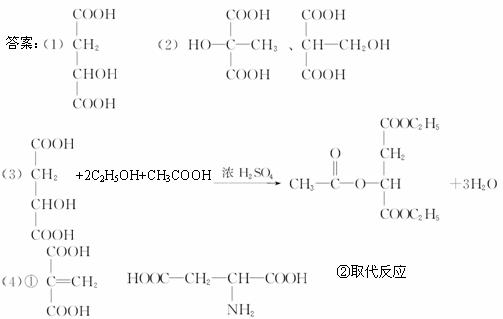
��֪BΪ��Ԫ��״�ṹ��C��ʹ��ˮ��ɫ��E��������嵰���ʵİ�����֮һ�������ʽΪC4H7O4N��
��д����C������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ_________��E�Ľṹ��ʽ_________��
��D![]() E�ķ�Ӧ������_________��
E�ķ�Ӧ������_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com