
��1���ڹ��ʵ�λ���У����ȵĵ�λ��______�������ĵ�λ��______��ʱ��ĵ�λ��______��ͬ����һ���������ʵ�����Ҳ�ǹ��ʵ�λ�е�һ������λ��______��
��2��д���������ʵ����ԭ����������Է���������
��Fe��______����Mg2����______����O2-��______����CO2��______��
��KCl��______����H2SO4��______��
��3�������ʵ�����������______������______����֮�ȡ��ڱ�ʾ�����ϣ������ǰٷ�����______��______��
�ڹ�����ܽ����һ���¶��£�ij������______g�ܼ���ﵽ����״̬ʱ���ܽ��������
������һ������������Һ�IJ����У�a.������b.���㣬c.��ȡ��d.�ܽ⡣��ȷ��˳����____________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
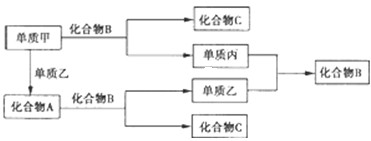
 ��֪A��B��DΪ��ѧ���������嵥�ʣ��ס��ҡ�������Ϊ�����Ļ������ˮ��Һ�ʼ��ԣ�����֮����������ʾת����ϵ�����ֲ��P��Ӧ��������ȥ��
��֪A��B��DΪ��ѧ���������嵥�ʣ��ס��ҡ�������Ϊ�����Ļ������ˮ��Һ�ʼ��ԣ�����֮����������ʾת����ϵ�����ֲ��P��Ӧ��������ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����ﳣ��������ͼ��ʾ��������������Ϊ��ͷ�ܷ�IJ����ܣ��м����һ�������̱���
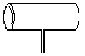 �ø������ɽ��ж���ʵ�飬����������װ�е⾧��ʱ���ô������ɽ��е�����ʵ�飬������װ�ü��������㡢�������ԡ�û����Ⱦ���ɷ���ʹ�õ��ŵ㡣��������пհף�
�ø������ɽ��ж���ʵ�飬����������װ�е⾧��ʱ���ô������ɽ��е�����ʵ�飬������װ�ü��������㡢�������ԡ�û����Ⱦ���ɷ���ʹ�õ��ŵ㡣��������пհף�
��1���ô��������ܷ������е�ʵ���� �� ����
A.NH4Cl�������ȷֽ�
B.KMnO4�������ȷֽ�
C.���ͺ�����һ���¶��»���ת��
D.��ˮCuSO4�͵����Ļ���ʵ��
��2������������װ��NO2��N2O4�Ļ������ʱ���ɷ������з�Ӧ�����Ի�ѧƽ��Ӱ���ʵ�顣��ʵ��IJ������̺�ʵ��������________________________��
��3�������������װ������ij��ɫ��Һ������ʱ��Һ����ɫ��dz����ȴ��ָ���ɫ������Һ������________________�������������װ������ij��ɫ��Һ��������Һʱ����Һ��Ϊ��ɫ����ȴ��ָ���ɫ�������Һ������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ȡ���������Լ��ֱ����6֧�Թ��У�������������ˮ�����Թܣ��۲쵽��������__________________________________________��
����������ʵĻ�ѧʽ������ʽ����_____________________________________________��
��2���ֱ�ȡδ�������Һ�������м��������Ѽ������Һ���۲쵽���������Ӧ�����ӷ���ʽ��
1֧�Թ����а�ɫ��������______________��
2֧�Թ�������ɫ��������______________��
����������ʵĻ�ѧʽ������ʽ����___________________________��
��3����������δ������ʵķ����۲쵽��������____________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com