
��1��A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӡ�BԪ��ԭ�������������Ǵ�����������2����DԪ���ǵؿ��ﺬ������Ԫ�ء�D��E��������֮��Ϊ24��F������������ˮ����Ϊ��ǿ�ᡣ
���ƶ�E��Ԫ�����ڱ��е�λ�ã���_______���ڣ�_______�塣
��A2D�ķе��A2E�ߵ�ԭ����_____________________________________________��
��E��F������������Ӧˮ���������(�����)�����ǿ��_______�������ʵ���Ũ�ȵ����������ʵ�ˮ��Һ��Ӧ������______________��
����101kPaʱ��1.4gBD������1.6gD2��������ȫȼ�գ�����BD2����ʱ�ų�14.15kJ
��������ʾBDȼ���ȵ��Ȼ�ѧ����ʽΪ��___________________________________��
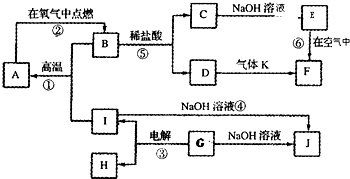
��ÿС��2�֣���1��������A ��2��H2O��H2O����֮�����������
��3������HClO4>H2SO4����Һ����H2SO4>HClO4��
��4��CO��g��+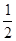 O2(g)=CO2��g������H=��283kJ/mol
O2(g)=CO2��g������H=��283kJ/mol
����������֪A���⡢B��̼��D���������Ƴ�E����F���ȣ�C����B��D֮�䣬��C�ǵ���ˮ�е������Ա�H2S����Ϊˮ���Ӽ����γ����������������ǿ���������ᣬ����Һ������ǿ������������Ũ�ȵĴ�С������c(H+)Խ������Խǿ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��2NO2��g��?N2O4��g����H��O���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ��ʾ��
��֪��2NO2��g��?N2O4��g����H��O���ں��º��������£���һ����NO2��N2O4�Ļ������ͨ���ݻ�Ϊ2L���ܱ������У���Ӧ�����и����ʵ����ʵ���Ũ��c��ʱ��t�ı仯��ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ���M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ���M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com