
| 0.224L |
| 22.4L/mol |
| 0.01mol��106g/mol |
| 2.0g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
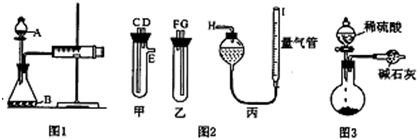
ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽�����������������װ�÷ֱ������Ca��NO3��2��Cu(NO3)2��AgNO3���ֹ��塣�����ȼ��г�װ��δ������
��1����ͬѧ���ȵ���Ca��NO3��2�����ȹ��̷��֣�װ�â��в��� ���ݣ�����ʯ����Һ��ѹ��װ�â��У��ô����ǵ�ľ��������е����壬ľ����ȼ������װ�â���ʣ��Ĺ����֪��ʣ������к���NԪ�أ����ԣ�3�ۡ���д��Ca��NO3��2���ȷֽ�����ɲ���Ļ�ѧʽ�� �� ��
��2����ͬѧ���ȵ���Cu(NO3)2�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ�������ʧ��ʯ����Һ��Ϊ��ɫ��Һ�弸������ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����д��Cu(NO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��
��3����ͬѧ���ȵ���AgNO3�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ��������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��ѧ��
![]() ����4AgNO3 2Ag2O��4NO2����O2����
����4AgNO3 2Ag2O��4NO2����O2����
![]() ����2AgNO3 2Ag��2NO2����O2����
����2AgNO3 2Ag��2NO2����O2����
������ȷ���� ����˵�����ɣ� ��
�������һ����ʵ��֤����Ľ�������ȷ�ģ� ��
��4��������3��ʵ��Ľ���������Ʋ����������ȷֽ�Ĺ��ɣ� ��
��5����������ͬѧ����������ag��������������ȫ�ֽ��ȡ��Ͳ���Ϊbml�����������ķֽ��ʣ�____________�����������������ϵ���ɲ��û���С����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
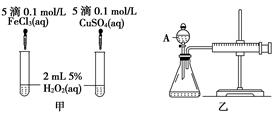
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6mol X�����0.6mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g) C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽�����������������װ�÷ֱ������Ca��NO3��2��Cu(NO3)2��AgNO3���ֹ��塣�����ȼ��г�װ��δ������
��1����ͬѧ���ȵ���Ca��NO3��2�����ȹ��̷��֣�װ�â��в��� ���ݣ�����ʯ����Һ��ѹ��װ�â��У��ô����ǵ�ľ��������е����壬ľ����ȼ������װ�â���ʣ��Ĺ����֪��ʣ������к���NԪ�أ����ԣ�3�ۡ���д��Ca��NO3��2���ȷֽ�����ɲ���Ļ�ѧʽ�� �� ��
��2����ͬѧ���ȵ���Cu(NO3)2�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ�������ʧ��ʯ����Һ��Ϊ��ɫ��Һ�弸������ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����д��Cu(NO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��
��3����ͬѧ���ȵ���AgNO3�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ��������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��ѧ��
![]() ����4AgNO3 2Ag2O��4NO2����O2����
����4AgNO3 2Ag2O��4NO2����O2����
![]() ����2AgNO3 2Ag��2NO2����O2����
����2AgNO3 2Ag��2NO2����O2����
������ȷ���� ����˵�����ɣ� ��
�������һ����ʵ��֤����Ľ�������ȷ�ģ� ��
��4��������3��ʵ��Ľ���������Ʋ����������ȷֽ�Ĺ��ɣ� ��
��5����������ͬѧ����������ag��������������ȫ�ֽ��ȡ��Ͳ���Ϊbml�����������ķֽ��ʣ�____________�����������������ϵ���ɲ��û���С����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012ѧ���Ĵ�ʡ��ɽ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g)  C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
| A�������ڵ�ѹǿ����ʱ����仯 |
| B����������ܶȲ�����ʱ����仯 |
| C��A���������ٸı� |
| D��ƽ���������ƽ����Է����������ٸı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012ѧ���Ĵ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��

(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g)  C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com