
A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
�Իش�
��1��EԪ�������ڱ��е�λ��Ϊ���������������������������� ����������
��2�� ��A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ �� ������������ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ������������������������ �����������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ�������������������������� ������
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ���������������������������������� ������
��5�� Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���⻯��DA���۵�Ϊ800�档DA����ˮ��Ӧ������������1mol DA��1 mol E���ʻ�ϼ���������ˮ����ַ�Ӧ�������������������� ������״���£���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 HO2-+H+
HO2-+H+ HO2-+H+
HO2-+H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
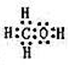
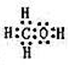
 H++HO2-
H++HO2- H++HO2-
H++HO2-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��EԪ�������ڱ��е�λ��Ϊ ��
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ ���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2�ͺ�ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ ��
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���Ȼ���DA���۵�Ϊ800��DA����ˮ��Ӧ������������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ�����ȫ������� ����״���£���
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡǦɽһ�е���У��һ��ѧ��������ѧ�Ծ����������� ���ͣ������
��14�֣�A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��EԪ�������ڱ��е�λ��Ϊ ��
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ ���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ ��
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ�ֻ�����DA���۵�Ϊ800�棬DA����ˮ��Ӧ�ų�����������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ���������������� ����״���£���
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ȱ������и�����һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
�Իش�
��1��EԪ�������ڱ��е�λ��Ϊ���������������������������� ����������
��2�� ��A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ �� ������������ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ������������������������ �����������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ�������������������������� ������
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ���������������������������������� ������
��5�� Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���⻯��DA���۵�Ϊ800�档DA����ˮ��Ӧ������������1mol DA��1 mol E���ʻ�ϼ���������ˮ����ַ�Ӧ�������������������� ������״���£���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com