

 2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0���������������
2CO2��N2�ܹ��Է����У���÷�Ӧ�Ħ�H 0��������������� 4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1��
4N2(g)��6H2O(g) ��H����1627.2kJ?mol��1�� 5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1��
5N2(g)��6H2O(g) ��H����1807.0 kJ?mol��1�� 7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1��
7N2(g)��12H2O(g) ��H����2659.9 kJ?mol��1�� 2NO(g)�Ħ�H�� kJ?mol��1��
2NO(g)�Ħ�H�� kJ?mol��1��
 2CO2��N2�ܹ��Է����У����Ц�H��T��S��0���÷�ӦΪ�������ʵ�����С���ؼ���Ӧ����S��0����÷�Ӧ�Ħ�H��0����2�����ݸ�˹���ɣ��١��ڵ�
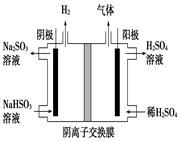
2CO2��N2�ܹ��Է����У����Ц�H��T��S��0���÷�ӦΪ�������ʵ�����С���ؼ���Ӧ����S��0����÷�Ӧ�Ħ�H��0����2�����ݸ�˹���ɣ��١��ڵ� 2NO(g)�Ħ�H��+179.8kJ?mol��1����3�������ȼ�ϵ��װ��ͼ֪��NO2�ڸ�������������Ӧ������N2O5,�缫��ӦʽΪ��NO2��NO3����e����N2O5����4������Fe��������⺬Cr2O72-�����Է�ˮ�������缫��ӦʽΪ��Fe - 2e-=Fe2+����������Cr2O72������ԭΪCr3+�����ӷ���ʽΪ
2NO(g)�Ħ�H��+179.8kJ?mol��1����3�������ȼ�ϵ��װ��ͼ֪��NO2�ڸ�������������Ӧ������N2O5,�缫��ӦʽΪ��NO2��NO3����e����N2O5����4������Fe��������⺬Cr2O72-�����Է�ˮ�������缫��ӦʽΪ��Fe - 2e-=Fe2+����������Cr2O72������ԭΪCr3+�����ӷ���ʽΪ

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
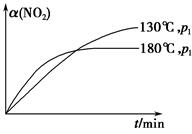
 N2(g)��CO2(g)��2H2O(g)����H����867 kJ��mol��1���÷�Ӧ���������������������Ⱦ����130 ���180 ��ʱ���ֱ�0.50 mol CH4��a mol NO2����1 L���ܱ������з�����Ӧ������й��������±���
N2(g)��CO2(g)��2H2O(g)����H����867 kJ��mol��1���÷�Ӧ���������������������Ⱦ����130 ���180 ��ʱ���ֱ�0.50 mol CH4��a mol NO2����1 L���ܱ������з�����Ӧ������й��������±���| ʵ�� ��� | �¶� | ʱ��/min | 0 | 10 | 20 | 40 | 50 |
| 1 | 130 �� | n(CH4)/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
| 2 | 180 �� | n(CH4)/mol | 0.50 | 0.30 | 0.18 | | 0.15 |
 4NO(g)��CO2(g)��2H2O(g)����H1����574 kJ��mol��1
4NO(g)��CO2(g)��2H2O(g)����H1����574 kJ��mol��1 2N2(g)��CO2(g)��2H2O(g)����H2
2N2(g)��CO2(g)��2H2O(g)����H2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ����1 | ȼú�м���ʯ��ʯ����SO2ת��ΪCaSO3��������ΪCaSO4 |
| ����2 | �ð�ˮ��SO2ת��ΪNH4HSO3��������Ϊ(NH4)2SO4 |
| ����3 | ��������ˮú����SO2��ԭΪS |
| ����4 | ��Na2SO3��Һ����SO2���ٵ��ת��ΪH2SO4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
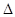 H= -Q1 kJ/mol��
H= -Q1 kJ/mol�� 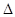 H= -Q2kJ/mol;
H= -Q2kJ/mol;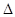 H= -Q3kJ/mol��
H= -Q3kJ/mol��| A��Q1��Q2��Q3 | B��Q1��Q3��Q2 | C��Q3��Q2��Q1 | D��Q2��Q1��Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
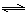 CO(g)+H2(g)����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
CO(g)+H2(g)����÷�Ӧ��ƽ�ⳣ������ʽΪ ��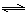 2CO��g�� ��H1
2CO��g�� ��H1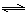 H2��g��+CO2��g�� ��H2
H2��g��+CO2��g�� ��H2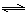 CO��g��+H2��g�� ��H3
CO��g��+H2��g�� ��H3  H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯�����ʾ��
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯�����ʾ��| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
 2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���� ��
2NO2(g) ��H��0���¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���� ��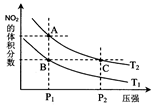
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NO2(g);��H=" +57.0" kJ��mol-1��NO2��N2O4��Ũ����ͼ����ʾ��NO2��N2O4��������������Ũ�ȵĹ�ϵ����ͼ��ʾ��
2NO2(g);��H=" +57.0" kJ��mol-1��NO2��N2O4��Ũ����ͼ����ʾ��NO2��N2O4��������������Ũ�ȵĹ�ϵ����ͼ��ʾ��
 2NO2(g) ��H=" +57.0" kJ��mol-1
2NO2(g) ��H=" +57.0" kJ��mol-1 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��-183 kJ��mol-1 | B��-91��5kJ��mol-1���� |
| C��+183kJ��mol-1������������������������ | D��+ 91��5kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2SO3��ƽ�ⳣ�����±���
2SO3��ƽ�ⳣ�����±���| �¶ȣ��棩 | 527 | 758 | 927 |
| ƽ�ⳣ�� | 784 | 1.0 | 0.04 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com