
��10�֣�A��B��C��D�ͼ�������ת����ϵ����֪���ʼ��Ƕ�����Ԫ����ɵ��Σ�����ij ������Һ����Ч�ɷ֣�����D�������ᡣ
������Һ����Ч�ɷ֣�����D�������ᡣ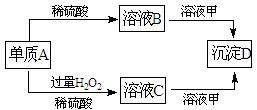
��ش��������⣺
��1�����A��Ԫ�������ڱ���λ�ڵ� ���ڵ� �塣
��2������C�������ӣ�������H+���IJ����������� ��
��3��Aת��ΪBʱ��ų���ɫ����E����298Kʱ1mol A��ȫ��Ӧ�ų�����QkJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4����Aת��ΪCʱ������ų���д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��5��д����Һ C����Һ��Ӧ�����ӷ���ʽ�� ��
C����Һ��Ӧ�����ӷ���ʽ�� ��
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
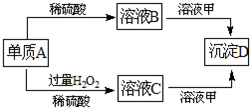 ��2011?Ϋ��ģ�⣩A��B��C��D�ͼ�������ת����ϵ����֪���ʼ��Ƕ�����Ԫ����ɵ��Σ�����ij������Һ����Ч�ɷ֣�����D�������ᣮ��ش��������⣺
��2011?Ϋ��ģ�⣩A��B��C��D�ͼ�������ת����ϵ����֪���ʼ��Ƕ�����Ԫ����ɵ��Σ�����ij������Һ����Ч�ɷ֣�����D�������ᣮ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
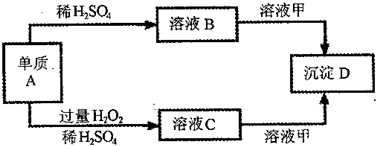
A��B��C��D�ͼ�������ת����ϵ����֪���Ƕ�����Ԫ���γɵ��Σ�����ij������Һ����Ч�ɷ֣�D�������ᡣ
��ش��������⣺
�����A��Ԫ�������ڱ���λ�ڵ� ���ڵ� ��
�Ƽ���ʹBѸ��ת��ΪD�����ǣ�д��ѧʽ�� ����ʱ����Һ���ֵ������� �� .
�Ǽ���C�������ӣ�������H+�����Լ� ������
��Aת��ΪBʱ��ų���ɫ����E����398Kʱ1molA��ȫ��Ӧ�ų�����QkJ�����
��Ӧ���Ȼ�ѧ����ʽΪ
����Aת��ΪCʱ������ų���д���÷�Ӧ�Ļ�ѧ����ʽ
��д����ҺC����Һ��Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�A��B��C��D�ͼ�������ת����ϵ����֪���ʼ��Ƕ�����Ԫ����ɵ��Σ�����ij������Һ����Ч�ɷ֣�����D�������ᡣ
��ش��������⣺
��1�����A��Ԫ�������ڱ���λ�ڵ� ���ڵ� �塣
��2������C�������ӣ�������H+���IJ����������� ��
��3��Aת��ΪBʱ��ų���ɫ����E����298Kʱ1mol A��ȫ��Ӧ�ų�����QkJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4�� ��Aת��ΪCʱ������ų���д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��5��д����ҺC����Һ��Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡΫ�����ظ������Խ�ѧ������⻯ѧ�Ծ� ���ͣ������
��10�֣�A��B��C��D�ͼ�������ת����ϵ����֪���ʼ��Ƕ�����Ԫ����ɵ��Σ�����ij������Һ����Ч�ɷ֣�����D�������ᡣ
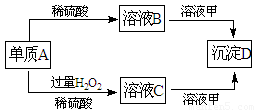
��ش��������⣺
��1�����A��Ԫ�������ڱ���λ�ڵ� ���ڵ� �塣
��2������C�������ӣ�������H+���IJ����������� ��
��3��Aת��ΪBʱ��ų���ɫ����E����298Kʱ1mol A��ȫ��Ӧ�ų�����QkJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4�� ��Aת��ΪCʱ������ų���д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��5��д����ҺC����Һ��Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com