
���� ��1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2��ʹ������ƿǰ������е�һ�������Ǽ�©��
��3������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��2����ʹ������ƿǰ������е�һ�������Ǽ������ƿ�Ƿ�©ˮ���ʴ�Ϊ���������ƿ�Ƿ�©ˮ��
��3����û��ϴ���ձ��Ͳ����������ʵ��������٣�Ũ��ƫС���ʢٴ���
��ת����Һʱ������������������ƿ���棬���ʵ��������٣�Ũ��ƫС���ʢڴ���
������ƿ�����������������ˮ����Һ��������䣬Ũ�Ȳ��䣬�ʢ۴���
�ܶ���ʱ���ӿ̶��ߣ���Һ�����ƫС��Ũ��ƫ�ߣ��ʢ���ȷ��
�ݶ��ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʢ���ȷ��
�ʴ�Ϊ���ܢݣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�������Լ����������ѶȲ���ע��ʵ��Ļ�������������ע�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2�ǻ�ԭ���� | |
| B�� | NaOH���������� | |
| C�� | Na2O2�У�-1�۵����ȵõ��ӣ���ʧ���� | |
| D�� | Na2O2����������ˮ�ǻ�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ϊʹˮ�����ʣ�����ˮ�����ڷ�����������Һ���ݹ��Ĺ����� | |
| B�� | PM2.5��������������������������� | |
| C�� | �ߴ�����̫���ܵ�ؼ���Ϣ���ٴ���������ҪӦ�� | |
| D�� | �Ӻ�ˮ����ȡ���ʲ�һ��Ҫͨ����ѧ��Ӧʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
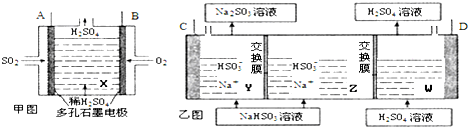
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Pb2++2CH3COO-+2H++S2-�TPbS��+2CH3COOH | |
| B�� | Pb2++H2S�TPbS��+2H+ | |
| C�� | Pb2++2CH3COO-+H2S�TPbS��+2CH3COOH | |
| D�� | ��CH3COO��2Pb+H2S�TPbS��+2CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
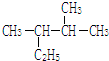 ��ϵͳ������������2��3-�������飻
��ϵͳ������������2��3-�������飻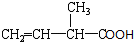 ���������ŵ��������Ȼ���
���������ŵ��������Ȼ���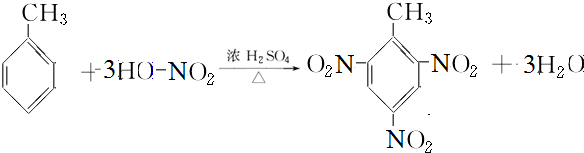 ��
�� ��
��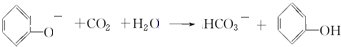 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KClO3��SO3����ˮ���ܵ��磬��KClO3��SO3Ϊ����� | |
| B�� | 25��ʱ���ô�����Һ�ζ���Ũ��NaOH��Һ��pH=7��V������VNaOH | |
| C�� | ������ˮ�еμ�ŨH2SO4��KW���� | |
| D�� | NaCl ��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ͷ�뵽Na2SO4��Һ�� | B�� | BaCl2��NaHSO4��Һ��Ӧ | ||
| C�� | С�մ���Һ�ͳ���ʯ��ˮ��Ӧ | D�� | Na2O2��CuSO4��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3���������������� | B�� | ƽ��������Ӧ�����ƶ� | ||
| C�� | ƽ�����淴Ӧ�����ƶ� | D�� | ���淴Ӧ���ʶ����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com