
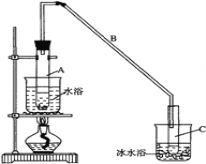
����Ŀ��ij��ѧС�������ͼװ�ã��Ի������Ʊ�����ϩ��
�ܶȣ�g/cm3�� | �۵㣨���� | �е㣨���� | �ܽ��� | |
������ | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | ��103 | 83 | ������ˮ |
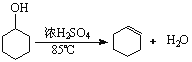
��֪��
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ��
���Թ�C�ڵõ�����ϩ��Ʒ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� _____________________________________��
�ڵ���B���˵�������е�������____________��A�����Ƭ�������� ___________��
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��______ �㣨����������������������Һ����___________________�������ţ�ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ��ͼװ��������ȴˮ��________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ������_________________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��________________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� _______
A������ʱ��70����ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_______
A�������Ը��������Һ B���ý����� C���ⶨ�е�
���𰸡���ֹ����ϩ�ӷ� ���� ������ �� C g ��ȥˮ�� 83C C BC
��������
��������Ũ��������¼��ȵ�85�����ɻ���ϩ��Ӧ����ˮԡ���ȣ������ܿ������������������ã����뻷��ϩ�еĻ��������������ʣ���Ҫ���з�Һ��Ȼ����̼������Һϴ�ӣ����ٲ�Ʒ�еĻ��������������ʣ��������ܺͽ����Ʒ�Ӧ��������ϩ���ܣ����߶��ܺ����Ը��������Һ��Ӧ��
��1���ٻ���ϩ�ķе�ϵͣ�Ϊ83�棬���ڱ�ˮԡ�п��Է�ֹ����ϩ�ӷ���
�ڳ������е�����������������ã�Һ���м������Ƭ�������Ƿ�ֹҺ����ȹ����б��У�
��2���ٻ���ϩ���ܶȱ�ˮС�����ϲ㣬��Һ����̼������Һϴ�ӣ�ϴȥ�������ʺͻ������ȣ�
������װ����ˮ���������½��ϳ�����g�ڽ���f�ڳ�����ʯ���ܺ�ˮ��Ӧ����������ʱ������ʯ���ܸ��
����Ϊ����ϩ�ķе�Ϊ83�棬���Կ��Ƶ��¶�Ӧ��83�����ң�
A.����ʱ��70�濪ʼ�ռ���Ʒ�����Ʒ�������ߣ�A����
B.������ʵ���������ˣ���ʹ��Ʒ�������ӣ�B����
C.�Ʊ���Ʒʱ���������Ʒһ����������ʹ���ɵĻ���ϩ�����٣�C��ȷ��
��ѡC��
��3������ϩ��Ʒ�к��������Ļ��������ʿ���ͨ���������Ĵ��ڣ�������ϩ�ľ�Ʒ�ʹ�Ʒ��
A.����ϩ�ͻ���������ʹ���Ը��������Һ��ɫ���ʲ���ͨ�����Ը������������ϩ�ľ�Ʒ�ʹ�Ʒ��A����
B.��������������Ʒ�Ӧ��������ϩ���ܣ������ý�����������ϩ�ľ�Ʒ�ʹ�Ʒ��B��ȷ��
C.�ⶨ�е�ķ������Լ���ϩ�ľ�Ʒ�ʹ�Ʒ��C��ȷ��
�ʺ����ķ���ΪBC��


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��MOH��ROH��Һ���ֱ��ˮϡ�������V��pH��![]() �ı仯��ͼ��ʾ����������������ǣ� ��
�ı仯��ͼ��ʾ����������������ǣ� ��

A. MOH�ļ���ǿ��ROH�ļ���
B. ROH�ĵ���̶ȣ�b�����a��
C. ������Һ����ϡ�ͣ������ǵ�c(OH��)���
D. ��![]() =2ʱ��������Һͬʱ�����¶ȣ���
=2ʱ��������Һͬʱ�����¶ȣ���![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����( )
A. ��������Һ������ȩ�е�ȩ����CH3CHO + 2Ag(NH3)2+ +2OH��![]() CH3COONH4 + 3NH3 + 2Ag��+ H2O
CH3COONH4 + 3NH3 + 2Ag��+ H2O
B. ��������Һ��ͨ������CO2��CO2+ H2O + 2C6H5O���� 2C6H5OH + CO32��
C. �������е���AgNO3��Һ����������Ԫ�أ�Cl��+ Ag+ ��AgCl��
D. ��������Һ�м�������İ�ˮ��Al3+ + 3NH3��H2O��Al(OH)3�� + 3NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
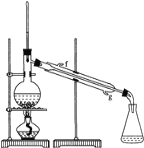
����Ŀ��������ʵ��װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���Ҳ�����ȷ����

A. ��ͼa��ʾװ������100mL0.100mol��L-1ϡ����
B. ��ͼb��ʾװ������FeCl3������Һ�Ʊ�FeCl3����
C. ��ͼc��ʾװ����ȡ����CO2����
D. ��ͼd��ʾװ�÷���CCl4��ȡ��ˮ���ѷֲ���л����ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ2NO2![]() 2NO+O2,�ں�ѹ�ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2NO+O2,�ں�ѹ�ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
�ٵ�λʱ��������n molO2��ͬʱ����2n molNO2
�ڵ�λʱ��������n molO2��ͬʱ����2n mol NO
��NO2��NO��O2 �����ʵ���Ũ��Ϊ2:2:1
�ܻ���������ɫ���ٸı��״̬ �ݻ��������ܶȲ��ٸı��״̬
A. �٢ۢ� B. �ڢۢ� C. �٢ܢ� D. �٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼú�������к��� SO2��Ϊ�����������������������ö��ַ���ʵ����������
��.(1)��ʪʽ���շ����������ռ��� SO2 ������Ӧ�Ӷ����������Լ����ʺ������÷����ռ�����_____(����ĸ���)��
a. ʯ���� b.CaCl2��Һ
(2)ij�������ú� SO2 ������������Cr2O72-�����Է�ˮ���������з�Ӧ��ĸ�Ԫ����Cr3+��ʽ���ڣ������������£�

���� SO2 ����������ˮʱ�������� SO2 ��_____�ԡ�
���������з�����Ӧ�����ӷ���ʽΪ_____��
��.ʯ��-ʯ�෨���ռ�dz��õ���������ʯ��-ʯ�෨�����շ�ӦΪCa(OH)2+SO2= CaSO3��+H2O�����ղ�����������ɹܵ���������������������ӦΪ2CaSO3+O2+4H2O =2CaSO4��2H2O����������ͼ��

�ռ�����շ�ӦΪ2NaOH+SO2=Na2SO3+H2O���÷����ص����������Ƽ���ǿ�����տ졢Ч�ʸߡ���������ͼ��

��֪��
�Լ� | Ca(OH)2 | NaOH |
�۸�(Ԫ/kg) | 0.36 | 2.9 |
���� SO2 �ijɱ�(Ԫ/mol) | 0.027 | 0.232 |
(3)ʯ��-ʯ�෨���ռ��ȣ�ʯ��-ʯ�෨���ŵ���_______��ȱ����_______��
(4)ijѧϰС����ʯ��-ʯ�෨���ռ�Ļ����ϣ����һ���Ľ��ġ���ʵ������ѭ������������������ͼ�еļס��ҡ�������_____��_____��_____(�ѧʽ)

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
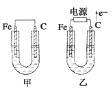
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�____________�����ҳ��е�____________����
���ҳ��������ĵ缫��Ӧʽ��___________________��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ_________________ ��
�ڼ׳���̼���ϵ缫��Ӧʽ��_____________________��
�����ҳ�ת��0.02 mol e����ֹͣʵ�飬��Һ�����200 mL������Һ��Ͼ��Ⱥ��pH��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���A1.44g��ȫȼ������2.16g H2O�����ɵ�CO2ǡ����200mL 1mol/LKOH��Һ�����������Σ���A�������м����������ͼ����ͼ��

��1����д��A�����ʽ_____________��
��2����д��A�ķ���ʽ______________��
��3����A��һ�ȴ���ֻ��һ�֣�A�Ľṹ��ʽΪ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬AԪ�ص�ԭ�Ӱ뾶������ԭ������С�ģ�BԪ��ԭ�ӵ��������������ڲ��������2����CԪ�ص�����������ˮ���������⻯���ܷ������Ϸ�Ӧ����һ���Σ�DԪ���ǵؿ��к�����ߵ�Ԫ�ء��ش��������⣺
(1)CԪ�ص�������____�������ڱ��е�λ����________��
(2)������BD2�Ľṹʽ��_________��������EA�ĵ���ʽ��___________��
(3)A��D��E����Ԫ���γɵĻ������к��еĻ�ѧ��������_________��
(4) D��EԪ�طֱ��γɵļ����Ӱ뾶��С��ϵ��_____(�����ӷ��ű�ʾ)��B��CԪ�طֱ��γɵļ���̬�⻯����ȶ��Դ�С��ϵ��___________(�û�ѧʽ��ʾ)��
(5)CԪ�صļ���̬�⻯������պ��Ũ����IJ�������������_________����ԭ����_________(�û�ѧ����ʽ����)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com