
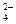 ��CO
��CO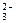 ��NO
��NO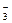 ��Cl����I�����ֽ�������ʵ��:
��Cl����I�����ֽ�������ʵ��:




















 ��1����֪��Һ��ǿ���ԣ�
��1����֪��Һ��ǿ���ԣ�
 ��2��ȡ�����������Ȼ�̼������������ˮ��CCl4��Ϊ�Ϻ�ɫ��
��2��ȡ�����������Ȼ�̼������������ˮ��CCl4��Ϊ�Ϻ�ɫ��
 ��3����ȡ���μ�ϡNaOH��Һ, ʹ��Һ��Ϊ����, �˹����������ɣ�
��3����ȡ���μ�ϡNaOH��Һ, ʹ��Һ��Ϊ����, �˹����������ɣ�
 ��4��ȡ��������������Һ, ��Na2CO3��Һ���ְ�ɫ������
��4��ȡ��������������Һ, ��Na2CO3��Һ���ְ�ɫ������
 ��5����ʵ�飨3���еļ�����Һ����, ������ų�, ��������ʹʪ��ɫʯ����ֽ������
��5����ʵ�飨3���еļ�����Һ����, ������ų�, ��������ʹʪ��ɫʯ����ֽ������
 �ɴ˿����ƶϣ�
�ɴ˿����ƶϣ�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | H+��Na+��A13+��Ag+��Ba2+ |
| ������ | OH-��C1-��CO32-��NO3-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��OH-��Na+��K+��MnO4- | B��H+��Cl-��Ba2+��NO3- |
| C��K+��Cl-��Na+��SO42- | D��NH4+��Mg2+��Cl-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ɫ��Һ�У�NH4+��Ag+��PO43-��Cl���ܴ������� |
| B��ˮ���������c��H+��=10-12 mol��L-1����Һ��K+��Ba2+��Cl?��Br-һ���ܴ������� |
| C��ʹ��ɫʯ����Һ������Һ��Fe2+��Mg2+��NO3-��Cl- �ܴ������� |
| D��pH��4.5�ķ���֭��c��H+����pH�� 6.5��ţ����c��H+����2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cu(OH)2��H2SO4 | B��Ba(OH)2��H2SO4 |
| C��Fe(OH)3��HCl | D��KHSO4��KOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ���е����ӣ���������Һ�д���������� �� ��
���е����ӣ���������Һ�д���������� �� ��A��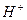  �� �� �� ��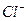 �� �� | B�� �� �� �� �� �� �� |
C�� �� ��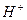 �� �� �� �� | D�� �� �� �� ��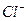 �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H����Na����CO32����MnO4�� | B��Cu2����K����NO3����SO42�� |
| C��K����H����NO3����OH�� | D��Mg2����Ca2����Cl����NO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe2+��Na+��NO3-��Cl- | B��Ba2+��Na+��NO3-��Cl- |
| C��SO42-��SO32-��NH4+��Na+���� | D��Mg2+��Na+��Br-��AlO2- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com