
| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�� ���� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
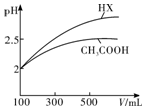
���� ��1��������Ӷ�Ӧ�������Խǿ�������ӵ�ˮ��̶�ԽС����Һ��pHԽС��
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ���������������ʵ�����С��Ũ�ȼ�С�����Լ�����ˮ�����ӻ��������䣬����ĵ���ƽ�ⳣ�����䣻
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��
��4����״���£���1.12L CO2ͨ��100mL 1mol•L-1��NaOH��Һ�У�1.12L CO2�����ʵ���Ϊ��$\frac{1.12L}{22.4L/mol}$=0.05mol���������Ƶ����ʵ���Ϊ��1mol•L-1��0.1L=0.1mol������ǡ����ȫ��Ӧ����̼���ƣ���Һ�д��ڵ���غ�������غ㣻
��5��CH3COOH��CH3COONa�Ļ����Һ�У����ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��CH3COO-����������Һ�еĵ���غ�������غ������㣮
��� �⣺��1��������Һ�����ʶ���ǿ�������Σ�ˮ��̶ȴ�СΪ��CO32-��ClO-��HCO3-��CH3COO-��ˮ����Լ��ԣ�ˮ��̶�Խ����Խǿ�����Լ���˳���ǣ�Na2CO3��NaClO��NaHCO3��CH3COONa��
��pH��С���������˳��Ϊ��CH3COONa��NaHCO3��NaClO��Na2CO3������a��d��c��b��
�ʴ�Ϊ��a��d��c��b��
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ�������Ũ�ȼ�С�����Լ�����
A��������Ũ�ȼ�С����A����
B����ˮϡ�����У����������ʵ���������������ʵ�����С������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$����B��ȷ��
C��ˮ�����ӻ��������䣬��C����
D��������Һ��ˮϡ��ʱ���Լ�����������Ũ�ȼ�С����������Ũ����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$����D��ȷ��
E������ĵ���ƽ�ⳣ�����䣬��E����
�ʴ�Ϊ��BD��
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��HX�ĵ���ƽ�ⳣ���ȴ����
�ʴ�Ϊ�����ڣ�
��4����״���£���1.12L CO2ͨ��100mL 1mol•L-1��NaOH��Һ�У�1.12L CO2�����ʵ���Ϊ��$\frac{1.12L}{22.4L/mol}$=0.05mol���������Ƶ����ʵ���Ϊ��1mol•L-1��0.1L=0.1mol������ǡ����ȫ��Ӧ����̼���ƣ�
����Һ�д��������غ㣺c��OH-��=c��H+��+c��HCO3-��+2c��H2CO3����
�ʴ�Ϊ��c��H+��+c��HCO3-����
��̼������Һ�д��ڵ���غ㣺c��H+��+c��Na+��=2c��CO32-��+c��HCO3-��+c��OH-����
�ʴ�Ϊ��2c��CO32-��+c��HCO3-��+c��OH-����
��5��CH3COOH��CH3COONa�Ļ����Һ�У����ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��CH3COO-��������c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=10-6mol/L-10-8mol/L=9.9��10-7mol/L��
�ʴ�Ϊ��9.9��10-7��
���� ���⿼�����ε�ˮ�⡢������ʵĵ��뼰��Ӱ�졢����ƽ�ⳣ����Ӧ�õȣ���Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ơ��ƿ��ƿ�����̷��������ĭ | |
| B�� | ��ʢ�ж���������������������������ܱ�����������ˮ�У����������ɫ��dz- | |
| C�� | ����ˮ�м�CaCO3����ҺƯ������ǿ | |
| D�� | 500�����ұ����¸������ںϳ�NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KSCN | B�� | BaCl2 | C�� | NaOH | D�� | NH3•H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KOH�ĵ���ʽ�� | B�� | ������̼�Ľṹʽ��O=C=O | ||
| C�� | CH4�����ģ�ͣ� | D�� | S2�������ӽṹʾ��ͼ��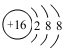 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͭ����Ʒ�Ʋ����������Ʒ������ǰ���������� | |
| B�� | ��״���£�22.4 L Cl2������NaOH��Һ��Ӧ��ת�Ƶ�����Ϊ2mol | |
| C�� | ˮ�����ӻ�����Kw�����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ | |
| D�� | Na2CO3��Һ�м�������Ca��OH��2���壬CO32-ˮ��̶ȼ�С����Һ��pH��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ˮ���а�ɫ�������ɣ���ԭ��Һһ����Al3+ | |
| B�� | FeCl3��CuCl2�Ļ����Һ�м������ۣ���ַ�Ӧ�����й���ʣ�࣬����KCSN��Һ���ܱ��Ѫ��ɫ | |
| C�� | п��Ũ�����Ӧ����ϡ���ᷴӦ�������� | |
| D�� | ����NaOH��Һ�����Ⱥ������������ʹʪ��ĺ�ɫʯ����ֽ��������ԭ��Һһ����NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N��M�����ܷ���������Ӧ | B�� | M�п���û�м� | ||
| C�� | N�ɷ�����ȥ��Ӧ | D�� | N�����к��м� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com