
����Ŀ����ͼ���������������л��������빦�ܹ�ϵͼ����ͼ�Ƕ��ǵ����ͼ�����ͼ�ش��������⡣

��1������ϸ���������Ԫ����_____________���������ں��������л���������ͼ�е�_______������ĸ��
��2����ͼ������D�Ļ���Ԫ�������________������C�IJ�ͬȡ����_________�IJ�ͬ��
��3��С������ϸ���У�����E��ָ__________________��
��4����ͬ������E��F���������ֽ⣬�������϶����_____________��
��5����ͼB��C�����ĵ��Ƿֱ���_______��___________(��д��������)������ˮ���������_____�ǣ����ɵ��ۡ���ά�ء���ԭ�ȶ��ǵĵ��嶼��____________________��
��6�����ۡ���ѿ�ǡ������ǡ���ԭ������ij�������������ijЩ�仯��������һ����________(������ֲ�)��
���𰸡�C G C H O N P R�� ���� F ���� ������ ���� ������ ����
��������
����ͼ��֪����ͼ�Ǻ��ᡢ�����ʡ�֬������������Ԫ�ء�������λ�����ࡢ�ֲ����ܣ�����E��A����Ҫ����Դ���ʣ������࣬E��A��ɣ�A�������ǣ�E�ǵ��ۻ���ԭ��F����Ҫ�Ĵ������ʣ�Ϊ֬����B��֬���Ļ�����ɵ�λ���ͺ�֬���G�����������Ҫ�е��ߣ�Ϊ�����ʣ�C�ǵ����ʵĻ�����ɵ�λ�����H���Ŵ���Ϣ��Я���ߣ�Ϊ���ᣬ������ɵ�λD�Ǻ�������ͼ���Ƕ��ǵ���ɣ���ѿ������2������������ɵģ����A�������ǣ���������1���������Ǻ�1���ӹ�����ɵģ����B�ǹ��ǣ���������1���������Ǻ�1���Ӱ�������ɣ����C�ǰ�������
��1������ϸ���������Ԫ����CԪ�أ��������ں��������л��������ǵ����ʣ���ͼ�е�G��
��2���ɷ�����֪��ͼ���е�D�Ǻ����ᣬ�������Ԫ����C��H��O��N��P������C�ǰ����ᣬ��ɵ����ʵİ��������R����ͬ��Ϊ20����
��3��С����������Ϊ��Դ���ʵĶ���E��Ҫ�ǵ�����
��4����E������ȣ�F֬���е�H�ĺ����϶࣬�����ֽ�ʱ���ĵ������϶���
��5���ɷ�����֪��B�ǹ��ǣ�C�ǰ����ǣ������Dz���ˮ����ǣ����ۡ���ά�ء���ԭ�ȶ��ǵĵ��嶼����������
��6��������������ѿ��������������ԭ������֪�������ܺϳ���ԭ�����һ���Ƕ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У�Ħ�����������ǣ� ��
A.10mL H2O
B.0.8mol NaOH
C.54 g Al
D.1 g H3PO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�й��������ӵļ�Ҫ����ͼ������������ȷ���ǣ�������
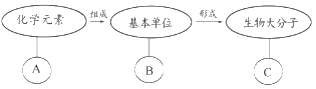
A. ��BΪ�����ǣ���C�ڶ���ϸ���п���Ϊ����
B. ��CΪRNA����BΪ���Ǻ����ᣬAΪC��H��O��N
C. ��C������Ϣ���ݡ����䡢���ȹ��ܣ���B����Ϊ������
D. ��BΪ���Ǻ����ᣬ��C���ܴ����������塢Ҷ���塢Ⱦɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и����������ܹ��Ի�ѧ��Ӧ�ٶ���������õ��ǣ� ��
A.�¶�
B.Ũ��
C.����
D.��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ1��ʾ�ĵ�ѭ������̬ϵͳ����ѭ������Ҫ��ɲ��֣������Ӿ��˵�ѭ���е�����ת����
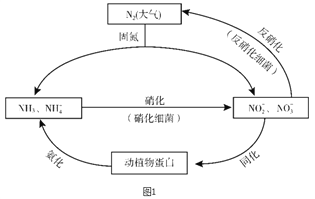
��1�������ͼ�ж�����˵����ȷ����________������ĸ��ţ���
A. �̵������У�N2ֻ��������
B. ������ϸ�������·���������������Ҫ������������
C. �����������������ֲ��˹��̵��Ե�ѭ����ɵ�Ӱ��
D. ͬ�������������У���Ԫ�ؾ�������ת�����л���
��2�����������У�NH3ת����HNO2�ķ�Ӧ�Ļ�ѧ����ʽΪ_______��
��3�������������У�CH3OH����Ϊ��Ӧ�Ļ�ԭ�����뽫�÷�Ӧ�����ӷ���ʽ����������5CH3OH + 6NO3- ![]() N2�� + 4HCO3- +��______+��
N2�� + 4HCO3- +��______+��
��4�������±����ݽ��й��㣬д����ҵ�ϳɰ���Ӧ���Ȼ�ѧ����ʽ��_______��
���ۼ� | N��N | H��H | N��H |
�Ͽ�1mol���ۼ�����������kJ�� | 946 | 436 | 391 |
��5����ⷨ�ϳɰ�����ԭ��ת���ʴ������ߣ��������洫ͳ�Ĺ�ҵ�ϳɰ����ա���ⷨ�ϳɰ�������ԭ����װ����ͼ2��ͼ3��ʾ��
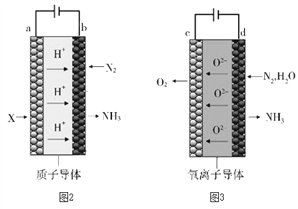
��ͼ2�У�a�缫��ͨ���XΪ_______��
��ͼ3�У�d�缫�ϵĵ缫��ӦʽΪ_______��
����ͼ2��ͼ3װ�õ�ͨ��ʱ����ͬ������ǿ����ȣ����Ч�ʷֱ�Ϊ80%��60%��������װ���в������������ʵ���֮��Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����. �ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʣ�������������������ʾ��
������ | NH4+��Mg2+��Ba2+ |
������ | OH����NO3����Cl�� |
ȡ�����������ֻ�����������ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)>c(��)>c(��)��
��1������_________________
��2��������__________________�����ʵ��ȷ�����������_______________________���������������ȷ������˿ղ��
��. ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ���Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
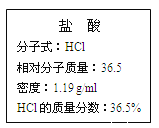
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________��
��2��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.40 mol/L��ϡ���ᡣ
�ٸ�ѧ������Ͳ��ȡ________mL����Ũ����������ơ�
�������ƹ����У�����ʵ��������������������ʵ���Ũ���к�Ӱ�죿���ڿո����� ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ʱ���ӹ۲�_________��
���ݺ���ҡ�ȡ����ú���Һ���½����ټ�����������ˮ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. ʯ�͵��ѻ���ú��������Һ�������ڻ�ѧ�仯����ʯ�͵ķ�����ú�ĸ������������仯
B. ![]() ��ϵͳ������Ϊ2��5-����-4-�һ�����
��ϵͳ������Ϊ2��5-����-4-�һ�����
C. ������һ��ʱ������ͼ�ȩ�����Ժ��ֱ�����ϣ���ȫȼ�����������������
D. ��֬�����ۡ������ʵȸ߷��ӻ����ﶼ���ڻ���һ�������¾��ɷ���ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������{[CH3CH(OH)COO]2Fe��3H2O}��һ�ֺܺõ�ʳƷ��ǿ����,������ˮ,����Ч���������ã�����������FeCO3��Ӧ�Ƶ�:
2CH3CH(OH)COOH+FeCO3+2H2O��[CH3CH(OH)COO]2Fe��3H2O +CO2����
I.�Ʊ�̼������:װ����ͼ��ʾ��
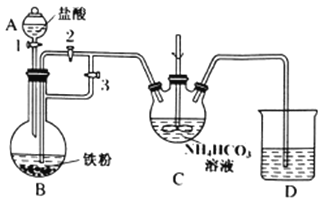
(1)C��������___________��
(2)��ϴ����,���װ��������,A�м�������,B�м�������,C�м���NH4HCO3��Һ��Ϊ˳�����ʵ��Ŀ��,����װ���л����Ĵر�˳��Ϊ:�رջ���______������______���������������,�رջ���1,��Ӧһ��ʱ���,�رջ���____,����_____��C�з����ķ�Ӧ�����ӷ���ʽΪ_______________��
��.�Ʊ�������������:
���Ƶõ�FeCO3����������Һ��,������������,��75���½���ʹ֮��ַ�Ӧ��Ȼ���ټ����������ᡣ
(3)�����������۵�������______________����������Һ�л�������������������ʵ������Ǹ�����������������________�����
��.�����������崿�ȵIJ���:
(4)����KMnO4�ζ����ⶨ��Ʒ��Fe2+�����������㴿��ʱ,���ֽ�����Ǵ���100%,��ԭ�������_____________________��
(5)����������,����Ce(SO4)2����Һ�ζ����вⶨ����Ӧ��Ce4+���ӵĻ�ԭ����ΪCe3+���ⶨʱ,�ȳ�ȡ5.760g��Ʒ,�ܽ����б�Ҫ������������ƿ���Ƴ�250mL��Һ,ÿ��ȡ25.00mL,��0.1000mol/LCe(SO4)2����Һ�ζ����յ�,��¼�������±���
�ζ����� | 0.1000mol/LCe(SO4)2����Һ���/mL | |
�ζ�ǰ���� | �ζ������ | |
1 | 0.10 | 19.85 |
2 | 0.12 | 21.32 |
3 | 1.05 | 20.70 |
���Ʒ��������������Ĵ���Ϊ_________(������������ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʯ�Ͳ�Ʒ�г�����H2S�⣬�����и�����̬���л�����COS��CH3SH���ش���������:
��1��CH3SH(����)�ĵ���ʽΪ________��
��2��һ��������Ϊ:���K2CO3������˹����
��K2CO3��Һ����H2S�ķ�ӦΪK2CO3+H2S=KHS+KHCO3���÷�Ӧ��ƽ�ⳣ���Ķ���ֵΪlgK=_____(��֪:H2CO3 lgK1=-6.4��lgK2=-10.3��H2SlgK1=-7��lgK2=-19)��
����֪�����Ȼ�ѧ����ʽ:
a.2H2S(g)+3O2(g)=2SO2(g)+ 2H2O(l) ��H1=-1172kJ/mol
b.2H2S(g)+O2(g)=2S(s)+2H2O(l) ��H2=-632kJ/mol
����˹��������ķ�ӦΪSO2��H2S���巴Ӧ����S(s)����÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��3��Dalleska�����о�������ǿ����Һ�п���H2O2����COS�����ѳ���Ӧ�Ļ�ѧ����ʽΪ______________��
��4��COSˮ�ⷴӦΪCOS(g)+H2O(g)![]() CO2(g)+H2S(g) ��H=-35.5kJ/mol��
CO2(g)+H2S(g) ��H=-35.5kJ/mol��
�û�����-Al2O3����������������ͬʱ���ı䷴Ӧ�¶ȣ����COSˮ��ת������ͼ1��ʾ��ij�¶�ʱ���ں����ܱ�������Ͷ��0.3molH2O(g)��0.1molCOS��COS��ƽ��ת������ͼ2��ʾ��
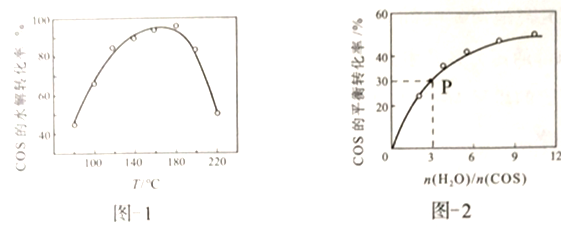
��ͼ1������-Al2O3��ˮ�⣬���¶�����ת������������ּ�С�Ŀ���ԭ����________��
����ͼ2��֪��P��ʱƽ�ⳣ��ΪK=______(������)��
��������-Al2O3��ˮ�⣬Ϊ���COS��ת���ʿɲ�ȡ�Ĵ�ʩ��____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com