������£�±�����봼��Ӧ�����ѣ�R-O-R�䣩��R-X+R��OH
R-O-R��+HX������A�������IJ���Ӧ�ɵõ������ܼ����� ૣ���Ӧ��ͼ��ͼһ��ʾ��
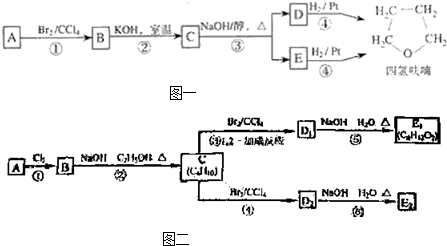
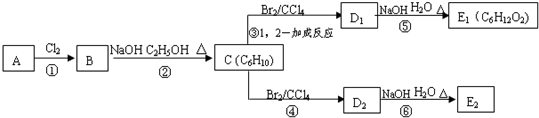
��ش��������⣺
��1��1mol A��1mol H
2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��Y��Y��̼Ԫ�ص���������ԼΪ65%����Y�ķ���ʽΪ
C4H10O
C4H10O
��A���������������ŵ�������
�ǻ���̼̼˫��
�ǻ���̼̼˫��
��A�Ľṹ��ʽΪ
CH2=CHCH2CH2OH
CH2=CHCH2CH2OH
��
��2���ڢ٢ڲ���Ӧ���ͷֱ�Ϊ��
�ӳɷ�Ӧ
�ӳɷ�Ӧ
����
ȡ����Ӧ
ȡ����Ӧ
��
��3��������B���еĻ�ѧ���ʣ���д��ĸ���ţ���
abc
abc
��
a���ɷ���������Ӧ b��ǿ���ǿ�������¾��ɷ�����ȥ��Ӧ
c���ɷ���������Ӧ d���������¿ɷ����Ӿ۷�Ӧ
��4��д��C��D��E�Ľṹ��ʽ��C
��D
��E
��
��5��д��������C��NaOHˮ��Һ��Ӧ�Ļ�ѧ����ʽ��
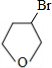
+NaOH
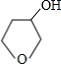
+NaBr

+NaOH

+NaBr
��
��6��д���������״���������ͬ���칹��Ľṹ��ʽ��
CH2=CHOCH2CH3��CH2=CHCH2OCH3��CH3CH=CHOCH3��CH2=C��CH3��OCH3
CH2=CHOCH2CH3��CH2=CHCH2OCH3��CH3CH=CHOCH3��CH2=C��CH3��OCH3
��
��ij�������A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź������ױ���������ֻ��һ�����͵��⣮
��1��A�Ľṹ��ʽΪ
��CH3��2C=C��CH3��2
��CH3��2C=C��CH3��2
��
��2��A��̼ԭ���Ƿ���ͬһƽ�棿
��
��
����ǡ����ǡ�����
��3����ͼ���У�D
1��D
2��Ϊͬ���칹�壬E
1��E
2��Ϊͬ���칹�壮
��Ӧ�ڵĻ�ѧ����ʽΪ
��CH
3��
2C��Cl��C��Cl����CH
3��
2+2NaOH
CH
2=C��CH
3��-C��CH
3��=CH
2+2NaCl+2H
2O
��CH
3��
2C��Cl��C��Cl����CH
3��
2+2NaOH
CH
2=C��CH
3��-C��CH
3��=CH
2+2NaCl+2H
2O
��C �Ļ�ѧ������
2��3-����-1��3-����ϩ
2��3-����-1��3-����ϩ
��E
2�Ľṹ��ʽ��
HOCH2C��CH3��=C��CH3��CH2OH
HOCH2C��CH3��=C��CH3��CH2OH
���ܡ��ķ�Ӧ����������
�ӳɷ�Ӧ
�ӳɷ�Ӧ
��
ȡ����Ӧ
ȡ����Ӧ
��



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
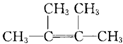
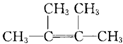

 +2NaOH
+2NaOH +2NaCl+2H2O
+2NaCl+2H2O +2NaOH
+2NaOH +2NaCl+2H2O
+2NaCl+2H2O
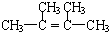
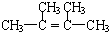











 +NaOH
+NaOH +NaBr
+NaBr +NaOH
+NaOH +NaBr
+NaBr