
| A����250mL����ƿ�ж��ݳ�250mL�ռ���Һ�� |
| B���ü�ʽ�ζ�����ȡ25mL�ռ���Һ����ƿ�в��μӼ��μ�����ָʾ���� |
| C������ƽ��ȷ��ȡ�ռ���Ʒmg�����ձ��м�����ˮ�ܽ⣻ |
| D�������ʵ���Ũ��ΪC mol��L-1�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶�ΪV1mL�� |
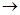
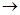
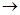 D
D 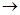 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������� | B�������� | C���ʼ��� | D�����ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ�еμ�ŨH2SO4��KW���� |
| B��CaCO3������ϡ���ᣬҲ�����ڴ��� |
| C����Na2Sϡ��Һ�У�c(H��)��c(OH��)��2c(H2S)��c(HS��) |
| D��NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�0.01mol��L-1������ҺpH=2 |
| B��95�洿ˮ��pH<7��˵�����ȿɵ���ˮ������ |
| C�������£���pH=3�Ĵ�����Һϡ����10������ҺpH=4 |
| D�������£���0.2 mol��L-1������������ˮ��Ϻ���ҺpH=1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢ� | B���ڢۢݢߢ� | C���ݢޢߢ� | D���ۢܢݢޢߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ۢ� | C���ڢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢ� | B���ۢݢޢ� | C���ܢݢޢ� | D���٢ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���٢ڢ� | C���ۢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | I1 | I2 | I3 | I4 |
| �����ܣ�kJ/mol�� | 578 | 1817 | 2745 | 11578 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com