
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����⾫��ͭʱ�ǽ�����ת��Ϊ��ѧ�ܣ���·��ÿͨ��2mole-ʱ�������ͻ��ܽ�64��ͭ |
| B����⾫��ͭʱ����Ϊ��ͭ������Ϊ��ͭ���������е���ʲ���Ҫ���� |
| C�������������϶����������Ϊ��������������������������������Ϊ���� |
| D����ƹ����е��Һ��Ҫ���ϸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
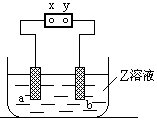
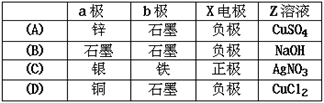
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D�� Cu2(OH)2CO3 Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
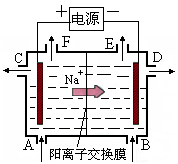
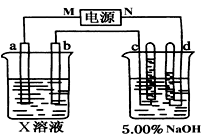
| A�������ĵ缫��ӦʽΪ4OH����4e��= 2H2O��O2�� |
| B����E�����ݳ���������H2 |
| C����D�����������ǽ�Ũ��NaOH��Һ |
| D����·��ת��4 mol����ʱ����������1 mol H2SO4���� |
�鿴�𰸺ͽ���>>
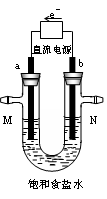
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O | B��HCl | C���������ƹ��� | D��̼��ƹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
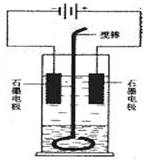
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
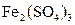 ��
�� �Ļ��Һ������֪
�Ļ��Һ������֪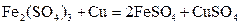 ������˵����ȷ����
������˵����ȷ����A��������ӦʽΪ��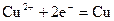 ������ ������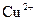 ����ʱ ����ʱ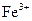 ���ŵ� ���ŵ� |
| B��������������������Ȼ������ͭ |
| C�������ϲ���������ͭ |
D�����ȱ�������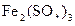 �� �� �������� �������� �� �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com