��2013?����һģ��I����һ���ݻ�Ϊ2L���ܱ������м���2molN
2��6molH
2���������·�Ӧ��
N
2��g��+3H
2��g��?2NH
3��g����H��0��5min��ﵽƽ�⣬���c��NH
3��=0.5mol/L��
��1���������´˷�Ӧ�Ļ�ѧƽ�ⳣ���ı���ʽK=
���¶����ߣ���Kֵ
��С
��С
������������С�����䡱��
��2���ӷ�Ӧ��ʼ��ƽ�⣬��H
2��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ��
0.15mol/��L?min��
0.15mol/��L?min��
��
��3����ƽ��ʱ������1mol N
2��3mol H
2������ͬ�¶����ٴ�ƽ��ʱc��NH
3��
��
��
0.25mol/L�������������������=����
��25�棬��0.4mol/LCH
3COOH��Һ��0.2mol/LNaOH��Һ��100mL��Ϻ�pH=5�������Ϻ���Һ�����Ϊ����Һ���֮�ͣ�
��1�������Һ������Ũ���ɴ�С��˳���ǣ�
c��CH3COO-����c��Na+����c��H+����c��OH-��
c��CH3COO-����c��Na+����c��H+����c��OH-��
��
��2����c��CH
3COO
-��+c��CH
3COOH��=
0.2
0.2
moL/L
��c��CH
3COO
-��-c��CH
3COOH��=
2����10-5-10-9��
2����10-5-10-9��
mol/L
����֪25��ʱ��K
sp[Fe��OH��
3]=8��10
-39�����¶��·�ӦFe��OH��
3+3H
+?Fe
3++3H
2O��ƽ�ⳣ��Ϊ
8��103
8��103
������ʽ�����㣩 ��0.001mob/L FeCl
3��Һ��ͨ�백��������仯���Բ��ƣ�����ʼ����ʱ��Һ��pHΪ
2.3
2.3
����lg5=0.7��




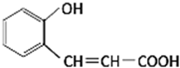 ��2013?����һģ����ͼ�Ǻϳ������㶹�ع��̵��м������ڸ����ʵ�˵������ȷ���ǣ�������
��2013?����һģ����ͼ�Ǻϳ������㶹�ع��̵��м������ڸ����ʵ�˵������ȷ���ǣ�������