
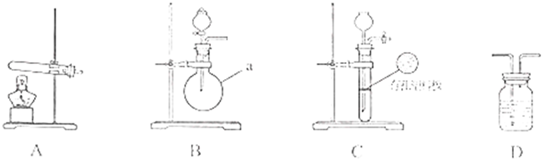
�ף��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�

��ͼA��B��CΪ�ף�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��

ʵ�鿪ʼǰװ���еĿ������ž�����С���ã���Ӧǰ����ͭ������Ϊ ������ͭ��Ӧ��ʣ����������Ϊ
������ͭ��Ӧ��ʣ����������Ϊ �����ɵ����ڱ�״���µ����
�����ɵ����ڱ�״���µ����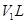 ����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
����С����ϴ��װ��Dǰ������������ɵ����ڱ�״���µ������
��1��д������a�����ƣ� ��
��2���ף�����С��ѡ���˲�ͬ������ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
|
|
ʵ��װ�� |
ʵ��ҩƷ |
�Ʊ�ԭ�� |
|
���� |
A |
�������ƣ������ |
��Ӧ�Ļ�ѧ����ʽΪ �� |
|
���� |
�� |
Ũ��ˮ���������� |
�û�ѧƽ��ԭ�������������Ƶ����ã� ��
|
��3����С�����������ݼ�����������е������ԭ�Ӹ���֮��Ϊ ��
��4���ڲ����ͼ�����ȷ������£���С�����������ݼ�����������е������ԭ�Ӹ���������С������ֵ����ԭ������� ��
��5����С����ԭ��ʵ��Ļ�����������һ��װ��ҩƷ��ʵ������������ʵ�飬�ó�������ʵ��������ҩƷ�������� ��
��1����2�֣����ƿ
��2���٣�1�֣�(NH4)2SO4+Ca(OH)2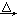 2NH3��+2H2O+CaSO4��2�֣� ��B��1�֣�
2NH3��+2H2O+CaSO4��2�֣� ��B��1�֣�
�ۣ�2�֣������������ڰ�ˮ����ȣ�����������Ũ�ȣ�ʹNH3+H2O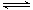 NH3��H2O
NH3��H2O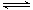 NH4��+OH�����淴Ӧ�����ƶ����ӿ찱���ݳ���2�֣�
NH4��+OH�����淴Ӧ�����ƶ����ӿ찱���ݳ���2�֣�
5V1:7(m1��m2) ��2�֣�
Ũ����������δ��Ӧ�İ������Ӷ�ʹ��������⺬��ƫ�ߣ�2�֣�
��5����ʯ�ң��������ƣ������Ƶȣ���2�֣�
��������
�����������2��Ũ��ˮ���������Ʋ�����ȣ��ܿ���Һ�������������ռ�ϴ�ѡB��
��3���⣺��H�����ʵ���Ϊy
nN2=V/22��4 nN= V/11��2 CuO--- Cu----O --------2H---- H2O
16 2
��m1��m2��y y= ��m1��m2��/8
�������ԭ�Ӹ���֮��Ϊ: V/11��2 : ��m1��m2��/8=5V1:7(m1��m2)
���㣺�����Ի�ѧʵ��Ϊ����������ʵ����ƣ�ʵ������������ͻ�ѧ�����֪ʶ��


 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
| ||
| ||
| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |||||||||
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� ��NH4��2SO4+Ca��OH��2
��NH4��2SO4+Ca��OH��2
| ||||||||
| ���� | �� B B |
Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� �����������ڰ�ˮ����ȡ�����������Ũ�ȣ�ʹNH3+H2O?NH3?H2O?NH4++OH-���淽���ƶ����ӿ찱���ݳ� �����������ڰ�ˮ����ȡ�����������Ũ�ȣ�ʹNH3+H2O?NH3?H2O?NH4++OH-���淽���ƶ����ӿ찱���ݳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

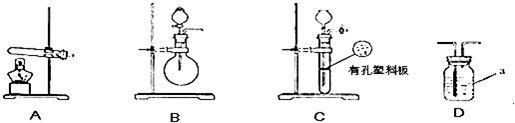
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����ͨ�ߵ�ѧУ����ȫ��ͳһ�������ۻ�ѧ���֣��Ĵ����������� ���ͣ�ʵ����
(17��)�ס��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ��������ʵ�����̣�
ʵ���У������Ƶĵİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��Ϻ�ɫ������ͭת��Ϊ��ɫ��ͭ��
��ͼA��B��CΪ�ס�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��
��С���ã���Ӧǰ����ͭ������m1g������ͭ��Ӧ��ʣ����������m2g�����ɵ����ڱ�״���µ����V1L��
��С���ã�ϴ��ǰװ��D������m3g��ϴ����װ��D������m4g�����ɰ����ڱ�״���µ����V2L��
��ش��������⣺
��1�������a������ ��
��2�����Aװ�������ԵIJ����� ��
��3���ס�����С��ѡ���˲�ͬ�ķ�����ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��Ŀո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ơ����ᡢ����� | ��Ӧ�Ļ�ѧ����ʽΪ �� �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com