
��Ԫ�����ڱ��д�������λ�õ�Ԫ���ڽṹ�����������������Ƶĵط����ڶ����ڵ�̼�������������������γ��⻯���Ԫ�ص��⻯���H![]() O�⣬����H
O�⣬����H![]() O
O![]() ��̼Ԫ�ص��⻯���CH
��̼Ԫ�ص��⻯���CH![]() �⣬����C
�⣬����C![]() H
H![]() �ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N
�ȣ���֮���Ƶĵ�Ԫ�ص��⻯����⣬����N![]() H
H![]() �ȡ�
�ȡ�
��1��̼ԭ��֮����Խ�ϳ���״�ṹ����ԭ��֮��Ҳ�����γ���״�ṹ�����赪ԭ�Ӽ�ֻ�Ե���������ʽ���ӳ���״�����γ��⻯����ϵ���⻯���ͨʽΪ ��
��2����ϵ���е�N![]() H
H![]() �ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N
�ǡ�����������ʱ������õ�Һ��ȼ�ϣ�Һ̬������������������������Һ̬ȼ�ϵ��ŵ��Dz�����������������Ⱦ����֪40g N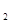 H
H![]() �ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�ڻ������ʱ��Ӧ�зų�710kJ������д���������ʱ�÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��3����ϵ�����е�NH![]() ��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
��ũҵ����ѧ��������ҵ������Ҫ���塣��ϳ�ԭ��Ϊ��
![]()
![]()
![]()
I����һ���¶��£���1.5molN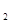 ��6 molH
��6 molH![]() ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
ͨ�뵽һ���̶��ݻ�ΪVL���ܱ������У�����Ӧ�ﵽƽ��ʱ�������������ѹǿΪ��ʼʱ��80%����
��ʱ��Ӧ�ų�������Ϊ kJ.
H![]() ��ת����= ��
��ת����= ��
���¶��ºϳɰ���Ӧ��ƽ�ⳣ��![]() = ��ֻ�����ֱ���ʽ��
= ��ֻ�����ֱ���ʽ��
II���ڱ����¶Ȳ��䣬��ͬ������ܱ������У�����ʼ�����ʵ�����ΪamolN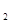 ��bmolH
��bmolH![]() ��cmolNH
��cmolNH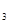 ,ƽ��ʱNH
,ƽ��ʱNH![]() �����ʵ�������Ϊ25%����
�����ʵ�������Ϊ25%����
�ﵽƽ��ʱ��I��II�ų������� ������ĸ���ţ�
A��һ�����
B��ǰ��һ��С�ں���
C��ǰ�ߵ��ڻ�С�ں���
D��ǰ�ߵ��ڻ���ں���
I��II�ϳɰ���ƽ�ⳣ���ֱ�Ϊ![]() ��
��![]() ��ͬ
��ͬ![]()
![]() �����������������=����
�����������������=����
��ʹ��ʼʱ��Ӧ������У�a��ȡֵ��ΧΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Ԫ�ر�� Ԫ������ |
�� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 |
| ��������ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | |
| ��ͻ��ϼ� | -2 | -3 | -1 | -3 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��18�֣���֪þ�ڿ�����ȼ����Ҫ��������þ��ͬʱ�����뵪����Ӧ�������ĵ���þ��Mg3N2�������ڱ��У�þ��ﮣ����������ڶԽ����ϣ����ڶԽ����ϵ�����Ԫ�������� �ƣ����Ϊ�Խ��߹��ݴ���ش�
(1) ��ڿ�����ȼ����Ҫ����ĵ���ʽ�� _______ͬʱ��������_____ _______����д��ѧʽ��
(2)�������������Ӧ��ˮ����Ļ�ѧʽ��_________________���������Ի����
֤����һ���۵��й����ӷ���ʽΪ______________________ _��__________ ______________��
(3)����֪��ӦBe2C��4H2O===2Be(OH)2��CH4������Al4C3��ǿ����Һ��Ӧ�����ӷ���ʽ
Ϊ________________________________________________��
(4) �õ���ʽ��ʾBeCl2���γɹ��̣�________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ����Э����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��18�֣���֪þ�ڿ�����ȼ����Ҫ��������þ��ͬʱ�����뵪����Ӧ�������ĵ���þ��Mg3N2�������ڱ��У�þ��ﮣ����������ڶԽ����ϣ����ڶԽ��� �ϵ�����Ԫ�������� �ƣ����Ϊ�Խ��߹��ݴ���ش�
�ϵ�����Ԫ�������� �ƣ����Ϊ�Խ��߹��ݴ���ش�
(1) ��ڿ�����ȼ����Ҫ����ĵ���ʽ�� _______ͬʱ��������_____ _______����д��ѧʽ��
(2)�������������Ӧ��ˮ����Ļ�ѧʽ��_________________���������Ի����
֤����һ���۵��й����ӷ���ʽΪ______________________ _��__________ ______________��
(3)����֪��ӦBe2C��4H2O===2Be(OH)2��CH4������Al4C3��ǿ����Һ��Ӧ�����ӷ���ʽ
Ϊ________________________________________________��
(4) �õ���ʽ��ʾBeCl2���γɹ��̣�________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��18�֣���֪þ�ڿ�����ȼ����Ҫ��������þ��ͬʱ�����뵪����Ӧ�������ĵ���þ��Mg3N2�������ڱ��У�þ��ﮣ����������ڶԽ����ϣ����ڶԽ����ϵ�����Ԫ�������� �ƣ����Ϊ�Խ��߹��ݴ���ش�
(1) ��ڿ�����ȼ����Ҫ����ĵ���ʽ�� _______ ͬʱ��������_____ _______����д��ѧʽ��
(2)�������������Ӧ��ˮ����Ļ�ѧʽ��_________________���������Ի����
֤����һ���۵��й����ӷ���ʽΪ______________________ _��__________ ______________��
(3)����֪��ӦBe2C��4H2O===2Be(OH)2��CH4������Al4C3��ǿ����Һ��Ӧ�����ӷ���ʽ
Ϊ________________________________________________��
(4) �õ���ʽ��ʾBeCl2���γɹ��̣�________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��16�֣�A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������Aԭ�Ӻ��������ӣ�A��E��D��F�ֱ�ͬ���壬��B��D ����������֮��Ϊ2��3���Իش��������⣺
��1��AԪ�ص������� ��EԪ�������ڱ��е�λ���� ��
��2��C��D��F����̬�⻯���ȶ�����ǿ������˳���� ���ѧʽ����
��3��E����������D������ȼ�����ɵĻ�����ĵ���ʽ�� ��
��4��������X��Y����A��D��E��F����Ԫ����ɡ�
��X��Y������ ���������ӡ����ۡ�����
��X��Y��ˮ��Һ���Ϸ�����Ӧ�����ӷ���ʽΪ ��
��5��������E2F��ˮ��Һ�е���˫��ˮ��ϡ���ᣬ���ȣ��е������ɡ������ӷ�Ӧ����ʽΪ�� ��
��6����A2D�����У�ÿ�����������ڵ�4�������γ��������֪�þ���������ȣ�����ֱ�ӱ��ͬ�¶�����ʱ��Ҫ���յ������������þ���������ȣ���51 kJ/mol��������⣬���Ӽ仹���ڷ��»���(11 kJ/mol)����þ���������ġ����ܡ���____kJ/mol��
��������ԭ�Ӻ���������ֻ��H����A����Ԫ�ء�A��E����E��ԭ����������B��C��D�ģ�����Eֻ����Na��B��D ����������֮��Ϊ2��3����ΪD��ԭ������С��Na������Dλ�ڵڶ����ڡ���B��D ��������������2�� 3����C�Ͳ��ܴ��ڣ�����B��D ��������������4��6����B��C��D��O����C��N��F��S��
��1����
��2���ǽ�����Խǿ���⻯����ȶ��Ծ�Խǿ���ǽ�������O��N��S��
��3���Ƶ�ȼ�ղ����ǹ������ƣ��������Ӽ��ͷǼ��Լ���
��4�����ǻ��õĽ�����������H��O��Na��S�γɵĻ�����һ�������ӻ�������Ƿֱ�ΪNaHSO4��NaHSO3��
��5��Na2S��S�Ļ��ϼ۴�����ͼ�̬�����л�ԭ�ԣ���˫��ˮ���������ԣ����߷���������ԭ��Ӧ��
��6��ÿ��ˮ���������ڵ�4�������γ��������ƽ��ÿ��ˮ�����γɵ������2�����������������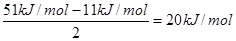 ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com