
 ���ʴ�Ϊ
���ʴ�Ϊ ��
��| VL |
| 22.4L/mol |
| VL |
| 22.4L/mol |
| VL |
| 22.4L/mol |


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
5KCl+KClO3+3H2O��

��ش��������⣺
��1��B�е�Һ����________����������________________________________________��
��2����֪C����װ����ˮ��D����װ����15mL30%KOH��Һ������ˮԡ���ȷ������ŵ���________________��ͨ��ʲô������Թ۲쵽KOH������Ӧ���___________________
__________________��
��3��E���ܿ�����һ����ɴ������Na2S2O3��Һ��ʪ���������������仹ԭ�����ն����Cl2����Ӧ�����ӷ���ʽΪ________________________��
��4����֪KCl��KClO3��ˮ�е��ܽ�����±���
�¶�/���������� 0���������������� 20���������������� 40���������������� 60���������������� 80���������������� 100
KCl/���������� 27.6������������ 34���������������� 40���������������� 45���������������� 51.1������������ 56.7
KclO3/g�������� 3.3������������ 7���������������� 14���������������� 24.5������������ 38.5������������ 57
��D����KOH������Ӧ��ʱ��ȡ���û����Һֱ�Ӽ���Ũ���������ȴ���Һ�������ľ�����________________������������ˮ���ȹ��ˣ�Ȼ����Һ��ȴ����ʱ�����ľ�����Ҫ��________________________������һ���ᴿKClO3��Ӧ���õķ�����________________
____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
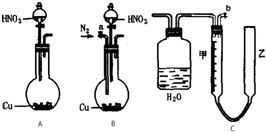
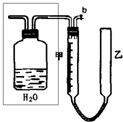
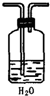
ʵ������KClO3����������ͼװ�á�ʵ���еĻ�ѧԭ��������MnO2��Ũ�����Ƶ�������Ȼ��ʹ�������ȵ�KOH��Һ��Լ70�棩��Ӧ��KClO3���仯ѧ����ʽΪ��3Cl2+6KOH![]()
5KCl+KClO3+3H2O��

��ش��������⣺
��1��B�е�Һ����________����������________________________________________��
��2����֪C����װ����ˮ��D����װ����15mL30%KOH��Һ������ˮԡ���ȷ������ŵ���________________��ͨ��ʲô������Թ۲쵽KOH������Ӧ���___________________
__________________��
��3��E���ܿ�����һ����ɴ������Na2S2O3��Һ��ʪ���������������仹ԭ�����ն����Cl2����Ӧ�����ӷ���ʽΪ________________________��
��4����֪KCl��KClO3��ˮ�е��ܽ�����±���
�¶�/���������� 0���������������� 20���������������� 40���������������� 60���������������� 80���������������� 100
KCl/���������� 27.6������������ 34���������������� 40���������������� 45���������������� 51.1������������ 56.7
KclO3/g�������� 3.3������������ 7���������������� 14���������������� 24.5������������ 38.5������������ 57
��D����KOH������Ӧ��ʱ��ȡ���û����Һֱ�Ӽ���Ũ���������ȴ���Һ�������ľ�����________________������������ˮ���ȹ��ˣ�Ȼ����Һ��ȴ����ʱ�����ľ�����Ҫ��________________________������һ���ᴿKClO3��Ӧ���õķ�����________________
____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����л�ѧϰ��1 ���ͣ�022
ʵ���ҿ�������ͼ��ʾװ��ģ�ҵ���ð��Ĵ������Ʊ����������ԭ�����ش��������⣺

(1)�����B��Ӧ��װ�ĸ������______________(������ѡ��ı��)��
[����]
A���Ȼ��ơ�������B����ʯ�ҡ�������C������������
(2)ʵ�������Ӧ��װ���г������ϵ�ͨ������Ŀ�����ԭ��Ϊ��_____________��д��C�������Ļ�ѧ��Ӧ�Ļ�ѧ����ʽ����˵��ʵ���жϿ����S��˿�ܼ������ֺ��ȵ�ԭ��_______________��Cװ���в�˿������״������ֱ��״��ԭ����_____________��
(3)��Ӧһ��ʱ�����D��������Һ����ֲ������������̣�E�еõ�ϡ���ᣬд��D��Һ̬���ʵ�����________________��
(4)ʵ�����������ֻ�Թֱܷ�ȡD��E�е�����Һ�壬�ֱ�����������ͭƬ��۲쵽��������_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������и�����ѧ�ڵ�һ���¿������ۣ���ѧ���� ���ͣ�ʵ����
��13�֣�ij�о���ѧϰС��������ͼ��ʾװ���о��Ҵ����������ķ�Ӧ����ش��������⣺

��1��װ�����Թ�B�������� ��
��2��ʵ���пɹ۲쵽ʯӢ��A�е�����Ϊ ��
��3����Ӧֹͣ��ȡ���Թ�C�ھƾ����ϼ��������ڣ��ɹ۲쵽�к�ɫ����������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��Ϊ�˲ⶨ��Ӧ��ʯӢ��A����������Ԫ�صĺ�������������ʵ�飺

��i�� ��������õ��IJ����������ձ�������������ͷ�ιܡ� ��
��ii�������йز���ܵIJ�����˵����ȷ���� ��
a���ζ���������ˮϴ�Ӻ����ֱ��װҺ
b����ƿ����Ҫ�ô���ҹ��ϴ
c���ζ������У��۾�ע�ӵζ�����Һ��仯
d���ζ�������30 s����Һ���ָ�ԭ������ɫ���ٶ���
��iii���ɿ�ͼ�����ݼ��㣬�ɵ�ʯӢ��A����������Ԫ�صİٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com