
AΓΔYΈΣ≥ΘΦϊΫπ τΒΞ÷ Θ§XΈΣ≥ΘΦϊΖ«Ϋπ τΒΞ÷ ΓΘ≥ΘΈ¬œ¬XΓΔGΓΔHΓΔIΈΣΤχΧεΘ§CΈΣ“ΚΧεΓΘB «”…»ΐ÷÷‘ΣΥΊΉι≥…ΒΡ―ΈΘ§Φ”»» ±ΖΔ…ζΖ÷Ϋβ…ζ≥…ΝΫ÷÷ΤχΧεΘ§ά以Κσ”÷Ω…Μ·ΚœΒΟΒΫBΓΘ”–ΙΊΈο÷ ÷°ΦδΒΡΉΣΜ·ΙΊœΒ»γœ¬ΆΦ(≤ΩΖ÷Ζ¥”ΠΧθΦΰΦΑ…ζ≥…Έο¬‘»Ξ)ΓΘ
«κΧν–¥œ¬Ν–Ω’ΑΉΘΚ
(1)BΒΡΒγΉ” ΫΈΣ____________________________ΘΜ
(2)œ÷”ΟA”κ ·ΡΪΉςΒγΦΪΘ§BΒΡ≈®»ή“ΚΉςΒγΫβ÷ »ή“ΚΘ§ΙΙ≥…‘≠Βγ≥ΊΓΘΤδ’ΐΦΪΖ¥”Π ΫΈΣ____________________ΘΜ
(3)Ζ¥”ΠΔόΒΡΜ·―ßΖΫ≥Χ ΫΈΣ__________________________Θ§Ζ¥”ΠΔή‘Ύ“±ΫπΙΛ“Β…œ τ”Ύ________________(ΧνΫπ τΒΡ“±ΝΕΖΫΖ®)ΘΜ
(4)¥”DΒΡΫαΨßΥ°ΚœΈο÷Τ±ΗDΒΡΈόΥ°ΨßΧεΒΡ≤ΌΉςΈΣ_____________________________ΘΜ
(5)Ζ¥”ΠΔΎΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________________________________________________ΘΜ
Ζ¥”ΠΔέΒΡάκΉ”ΖΫ≥Χ ΫΈΣ___________________________________________________ΓΘ
(1) 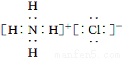
(2)2NH4+ΘΪ2eΘ≠=2NH3ΓϋΘΪH2Γϋ
(3)3FeΘΪ4H2O(g)  Fe3O4ΘΪ4H2ΓΓ»»ΜΙ‘≠Ζ®Μρ¬Ν»»ΜΙ‘≠Ζ®
Fe3O4ΘΪ4H2ΓΓ»»ΜΙ‘≠Ζ®Μρ¬Ν»»ΜΙ‘≠Ζ®
(4)ΫΪDΒΡΫαΨßΥ°ΚœΈο‘ΎHClΤχΝς÷–Φ”»»
(5)4NH3ΘΪ5O2 4NOΘΪ6H2OΓΓAl3ΘΪΘΪ3NH3ΓΛH2O=Al(OH)3ΓΐΘΪ3NH4+ [ΜρAl3ΘΪΘΪ3NH3ΘΪ3H2O=Al(OH)3ΓΐΘΪ3NH4+]
4NOΘΪ6H2OΓΓAl3ΘΪΘΪ3NH3ΓΛH2O=Al(OH)3ΓΐΘΪ3NH4+ [ΜρAl3ΘΪΘΪ3NH3ΘΪ3H2O=Al(OH)3ΓΐΘΪ3NH4+]
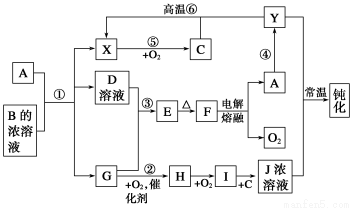
ΓΨΫβΈωΓΩΗυΨίΧβΗ…÷–ΧαΙ©–≈œΔΘ§CΈΣH2OΘΜBΈΣNH4ClΘ§Ϋπ τAΈΣΜνΤΟΫπ τ«“ΈΣΒγΫβΖ®÷Τ±Η ±”–O2…ζ≥…Θ§A”ΠΈΣAlΘ§Al”κNH4Cl»ή“ΚΖ¥”Π…ζ≥…X(H2)ΓΔD(AlCl3)ΓΔG(NH3)Θ§Υυ“‘HΈΣNOΘ§IΈΣNO2Θ§JΈΣHNO3ΓΘ
AlCl3ΚΆNH3Ζ¥”Π…ζ≥…Al(OH)3(E)Θ§FΈΣAl2O3Θ§≥ΘΈ¬œ¬‘Ύ≈®HNO3÷–ΕέΜ·ΒΡ≥ΐAlΆβΘ§ΜΙ”–FeΘ§Ά®Ιΐ¬Ν»»Ζ¥”ΠΔή…ζ≥…Fe(Y)Θ§ΧζΚΆH2O(g)Ζ¥”Π…ζ≥…H2ΓΘ
(2)AlΓΔ ·ΡΪΉςΒγΦΪΘ§NH4ClΈΣΒγΫβ÷ »ή“ΚΘ§ΙΙ≥…‘≠Βγ≥ΊΘ§’ΐΦΪ…œ―τάκΉ”ΒΟΒγΉ”Θ§ΒγΦΪΖ¥”Π ΫΈΣ2NH4+ΘΪ2eΘ≠=2NH3ΓϋΘΪH2ΓϋΓΘ
(4)¥”AlCl3ΨßΧε÷–÷Τ±ΗΈόΥ°AlCl3Θ§ΈΣΝΥΖά÷ΙΥ°ΫβΘ§”Π‘ΎHClΤχΖ’÷–Φ”»»ΓΘ


 ΟΩ»’10Ζ÷÷”ΩΎΥψ–ΡΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ
ΟΩ»’10Ζ÷÷”ΩΎΥψ–ΡΥψΥΌΥψΧλΧλΝΖœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Η¥œΑœό ±Φ·―Β Ή®Χβ10Ζ«Ϋπ τ‘ΣΥΊΒΞ÷ ΦΑΜ·ΚœΈοΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν– Β―ι ¬ ΒΥυΒΟ≥ωΒΡœύ”ΠΫα¬έΚœάμΒΡ «Θ®ΓΓΓΓΘ©
Β―ι ¬ ΒΫα¬έ
AΘ°Cl2ΒΡΥ°»ή“ΚΩ…“‘ΒΦΒγCl2 «ΒγΫβ÷
BΘ°ΫΪ»ΦΉ≈ΒΡΟΨΧθ…λ»κ Δ”–CO2ΒΡΦ·ΤχΤΩ÷–ΦΧ–χ»Φ…’ΜΙ‘≠–‘ΘΚMg>C
CΘ°SO2Ω…“‘ ΙΥα–‘KMnO4»ή“ΚΆ …ΪSO2ΨΏ”–Τ·ΑΉ–‘
DΘ°ΫΪΧζΖέΖ≈»κœΓHNO3÷–≥δΖ÷Ζ¥”ΠΚσΘ§ΒΈ»κKSCN»ή“ΚΈόΟςœ‘œ÷œσœΓHNO3≤ΜΡήΫΪFe―θΜ·≥…Fe3ΘΪ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °ΝυΈο÷ ΫαΙΙ”κ–‘÷ ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
“―÷ΣAΓΔBΓΔCΓΔDΓΔEΓΔFΈΣ‘ΣΥΊ÷ήΤΎ±μ÷–‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΒΡ«Α20Κ≈‘ΣΥΊΓΘA”κBΘ§CΓΔD”κEΖ÷±πΈΜ”ΎΆ§“Μ÷ήΤΎΓΘA‘≠Ή”L≤ψ…œ”–2Ε‘≥…Ε‘ΒγΉ”Θ§BΓΔCΓΔDΒΡΚΥΆβΒγΉ”≈≈≤ΦœύΆ§ΒΡΦρΒΞάκΉ”Ω…–Έ≥…“Μ÷÷C3DB6–ΆάκΉ”ΨßΧεXΘ§CEΓΔFAΈΣΒγΉ” ΐœύΆ§ΒΡάκΉ”ΨßΧεΓΘ
(1)–¥≥ωA‘ΣΥΊΒΡΜυΧ§‘≠Ή”ΦέΒγΉ”≈≈≤Φ Ϋ__________ΘΜFάκΉ”ΒγΉ”≈≈≤Φ ΫΈΣ__________ΓΘ
(2)–¥≥ωXΒΡΜ·―ß Ϋ__________________________Θ§Μ·―ßΟϊ≥ΤΈΣ_________________ΓΘ
(3)–¥≥ωX…φΦΑΫπ τ“±ΝΕ÷–ΒΡ“ΜΗωΜ·―ßΖΫ≥Χ Ϋ_______________________________ΓΘ
(4) ‘Ϋβ ΆΙΛ“Β“±ΝΕD≤Μ“‘DE3Εχ «“‘D2A3ΈΣ‘≠ΝœΒΡ‘≠“ρΘΚ
________________________________________________________________________ΓΘ
(5)CEΓΔFAΒΡΨßΗώΡήΖ÷±πΈΣ786 kJΓΛmolΘ≠1ΓΔ3 401 kJΓΛmolΘ≠1Θ§ ‘Ζ÷ΈωΒΦ÷¬ΝΫ’ΏΨßΗώΡή≤ν“λΒΡ÷ς“Σ‘≠“ρ «_______________________________________
(6)F”κBΩ…–Έ≥…άκΉ”Μ·ΚœΈοΘ§ΤδΨßΑϊΫαΙΙ»γΆΦΥυ ΨΘΚF”κB–Έ≥…άκΉ”Μ·ΚœΈοΒΡΜ·―ß ΫΈΣ________ΘΜΗΟάκΉ”Μ·ΚœΈοΨßΧεΒΡΟήΕ»ΈΣa gΓΛcmΘ≠3Θ§‘ρΨßΑϊΒΡΧεΜΐ «________________(÷Μ“Σ«σΝ–≥ωΥψ Ϋ)ΓΘ
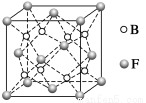
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °Εΰ≥ΘΦϊΖ«Ϋπ τ‘ΣΥΊΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–Έο÷ ΦδΡή÷±Ϋ”ΉΣΜ·ΒΡ‘ΣΥΊ «(ΓΓΓΓ)

AΘ°¬» BΘ°Νρ CΘ°¬Ν DΘ°Χζ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °Εΰ≥ΘΦϊΖ«Ϋπ τ‘ΣΥΊΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
ΓΑ…ώΨ≈Γ±Ζ…¥§…œ‘Ί”–Μν–‘ΧΩ≤ΡΝœΒΡΤς≤ΡΘ§ΤδΉς”Ο «(ΓΓΓΓ)
AΘ°¥Πάμ”νΚΫ‘±ΒΡ…ζΜνά§Μχ BΘ°»Ξ≥ΐΚτΈϋΖœΤχ÷–ΒΡ“λΈΕ
CΘ°ΧαΙ©…ζΜν÷––η“ΣΒΡΡήΝΩ DΘ°ΗΡ…Τ ß÷ΊΧθΦΰœ¬ΒΡΤΫΚβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °ΤΏ”–ΜζΜ·―ßΜυ¥ΓΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
¬»ΏΝΗώάΉ(clopidogrel,1) «“Μ÷÷”Ο”Ύ“÷÷Τ―Σ–ΓΑεΨέΦ·ΒΡ“©ΈοΘ§ΗυΨί‘≠ΝœΒΡ≤ΜΆ§Θ§ΗΟ“©ΈοΒΡΚœ≥…¬ΖœΏΆ®≥Θ”–ΝΫΧθΘ§Τδ÷–“‘2?¬»±ΫΦΉ»©ΈΣ‘≠ΝœΒΡΚœ≥…¬ΖœΏ»γœ¬ΘΚ
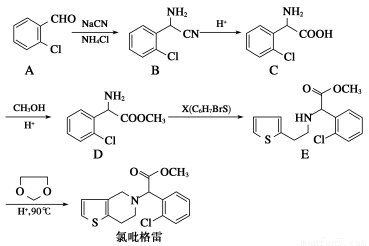
(1)Ζ÷Ή”D÷–ΒΡΙΌΡήΆ≈Οϊ≥ΤΈΣ________________ΓΘXΒΡΫαΙΙΦρ ΫΈΣ____________ΓΘ
(2)Ζ÷Ή”CΩ…‘Ύ“ΜΕ®ΧθΦΰœ¬Ζ¥”Π…ζ≥…“Μ÷÷≤ζΈοΘ§ΗΟ≤ζΈοΖ÷Ή”÷–Κ§”–3ΗωΝυ‘ΣΜΖΘ§–¥≥ωΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ______________________________________
(3)DΓζEΒΡΖ¥”Πάύ–Ά «________Ζ¥”ΠΓΘ
(4)–¥≥ωA τ”ΎΖΦœψΉεΜ·ΚœΈοΒΡΥυ”–Ά§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΘΚ__________ΓΘ
(5)“―÷ΣΘΚCO COROH
COROH COROR
COROR
‘ρ”…““¥ΦΓΔΦΉ¥ΦΈΣ”–Μζ‘≠Νœ÷Τ±ΗΜ·ΚœΈο Θ§–η“ΣΨ≠άζΒΡΖ¥”Πάύ–Ά”–__________(Χν–¥±ύΚ≈)ΓΘΔΌΦ”≥…Ζ¥”ΠΓΓΔΎœϊ»ΞΖ¥”ΠΓΓΔέ»Γ¥ζΖ¥”ΠΓΓΔή―θΜ·Ζ¥”ΠΓΓΔίΜΙ‘≠Ζ¥”ΠΘ§–¥≥ω÷Τ±ΗΜ·ΚœΈο
Θ§–η“ΣΨ≠άζΒΡΖ¥”Πάύ–Ά”–__________(Χν–¥±ύΚ≈)ΓΘΔΌΦ”≥…Ζ¥”ΠΓΓΔΎœϊ»ΞΖ¥”ΠΓΓΔέ»Γ¥ζΖ¥”ΠΓΓΔή―θΜ·Ζ¥”ΠΓΓΔίΜΙ‘≠Ζ¥”ΠΘ§–¥≥ω÷Τ±ΗΜ·ΚœΈο ΒΡΉνΚσ“Μ≤ΫΖ¥”Π_______________________________________________ΓΘ
ΒΡΉνΚσ“Μ≤ΫΖ¥”Π_______________________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®Χβ °“Μ≥ΘΦϊΫπ τ‘ΣΥΊΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
AΓΔBΓΔCΓΔDΓΔE «÷–―ßΫΉΕΈ≥ΘΦϊΒΡ5÷÷Μ·ΚœΈοΘ§AΓΔB «―θΜ·ΈοΘ§‘ΣΥΊXΓΔYΒΡΒΞ÷ «…ζΜν÷–≥ΘΦϊΒΡΫπ τΘ§œύΙΊΈο÷ ΦδΒΡΉΣΜ·ΙΊœΒ»γΆΦΥυ ΨΘΚ
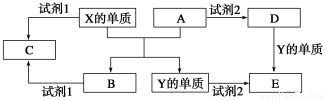
(1)XΒΡΒΞ÷ ”κAΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «_______________________________ΓΘ
(2)»τ ‘ΦΝ1 «NaOH»ή“ΚΘ§‘ρXΒΡΒΞ÷ ”κ ‘ΦΝ1Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «
___________________________________
(3)»τ ‘ΦΝ1ΚΆ ‘ΦΝ2Ψυ «œΓΝρΥαΓΘ
ΔΌΦλ―ιΈο÷ DΒΡ»ή“Κ÷–Ϋπ τάκΉ”ΒΡΖΫΖ® «___________________________________ΓΘ
ΔΎΫΪΈο÷ C»ή”ΎΥ°Θ§Τδ»ή“Κ≥ Υα–‘Θ§‘≠“ρ «(”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ)
________________________________________________ΓΘ
ΔέΡ≥ΗΏ–ßΨΜΥ°ΦΝ «”…Y(OH)SO4ΨέΚœΒΟΒΫΒΡΘ§ΙΛ“Β…œ“‘EΓΔœΓΝρΥαΚΆ―«œθΥαΡΤΈΣ‘≠Νœ÷Τ±ΗY(OH)SO4Θ§Ζ¥”Π÷–”–NO…ζ≥…Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «_____________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®ΧβΝυΈο÷ ΫαΙΙΚΆ‘ΣΥΊ÷ήΤΎ¬…ΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
“―÷ΣΕΧ÷ήΤΎ‘ΣΥΊΦΉΓΔ““ΓΔ±ϊΓΔΕΓΓΔΈλΒΡ‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΘ§Τδ«βΜ·Έο÷–ΦΉΓΔ““ΓΔ±ϊΓΔΕΓΓΔΈλΒΡΜ·ΚœΦέ»γœ¬Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
‘ΣΥΊ | ΦΉ | ““ | ±ϊ | ΕΓ | Έλ |
Μ·ΚœΦέ | Θ≠4 | ΘΪ1 | Θ≠4 | Θ≠2 | Θ≠1 |
AΘ°““ΒΡ≥ΘΦϊ―θΜ·Έο÷Μ”–“Μ÷÷
BΘ°ΤχΧ§«βΜ·ΈοΈ»Ε®–‘ΘΚ±ϊΘΨΕΓ
CΘ°±ϊΒΡ―θΜ·ΈοΡή”κΈλΒΡ«βΜ·ΈοΒΡΥ°»ή“ΚΖ¥”Π
DΘ°‘≠Ή”ΑκΨΕ¥σ–ΓΘΚΈλΘΦ±ϊ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2014ΗΏΩΦΜ·―ßΕΰ¬÷Ή®ΧβΆΜΤΤ Ή®ΧβΕΰΜ·―ß”Ο”οΦΑ≥Θ”ΟΦΤΝΩΝΖœΑΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
…ηNAΈΣΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
AΘ°0.44 g C3H8÷–Κ§”–ΒΡΙ≤ΦέΦϋΉή ΐΡΩΈΣ0.1NA
BΘ°±ξΉΦΉ¥Ωωœ¬Θ§2.24 L»ΐ¬»ΦΉΆι÷–Κ§”–ΧΦ¬»Ι≤ΦέΦϋΒΡ ΐΡΩΈΣ0.3NA
CΘ°25 Γφ ±Θ§1 L pHΘΫ12ΒΡNa2CO3»ή“Κ÷–Κ§”–NaΘΪΒΡ ΐΡΩΈΣ0.02NA
DΘ°1 mol±υ¥ΉΥαΚΆ1 mol““¥Φ‘ΎΦ”»»ΚΆ≈®ΝρΥαΧθΦΰœ¬≥δΖ÷Ζ¥”Π…ζ≥…ΒΡΥ°Ζ÷Ή” ΐΈΣNA
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com