
 ֮��Ϊ2:1
֮��Ϊ2:1 ��b��
��b�� ��c��
��c�� ��d��
��d�� ������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a
������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a ��Һ�еμ�NaOH��Һ����ҺpH=7����
��Һ�еμ�NaOH��Һ����ҺpH=7����

| A���ۢݢ� | B���ۢܢ� | C���ܢݢ� | D���٢ڢ� |
 ֮��Ϊ1:10������ȷ�������ε�ˮ��Һ���Լ��ԣ�ͬŨ�ȣ�����ǿ��˳��Ϊd>b>c>a,��pH��ȵ�������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a���ܴ���
֮��Ϊ1:10������ȷ�������ε�ˮ��Һ���Լ��ԣ�ͬŨ�ȣ�����ǿ��˳��Ϊd>b>c>a,��pH��ȵ�������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a���ܴ��� ��Һ�еμ�NaOH��Һ����ҺpH=7����c(Na) < 2c(SO42��)������ȷ������ȷ����3a=14ʱ�����ҺpH=7�����ϣ���������ѡ��ΪA��
��Һ�еμ�NaOH��Һ����ҺpH=7����c(Na) < 2c(SO42��)������ȷ������ȷ����3a=14ʱ�����ҺpH=7�����ϣ���������ѡ��ΪA��

 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����ȴ�����º���250mL����ƿ�ж������250mLϡ���ᡣ |
| B����ij������ȡ25.00mLϡ��������ƿ�в����뼸��ָʾ���� |
| C������ʽ�ζ��ܺͼ�ʽ�ζ���������ˮϴ�Ӹɾ������ø���ʢ��Һ��ϴ�� |
| D�������ʵ���Ũ��ΪM mol/L�ı�NaOH��Һװ���ʽ�ζ��ܣ�����Һ����¿�ʼ����ΪV1mL�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��V1��10V2 | B��V1��10V2 | C��V1��10V2 | D��V2��10V1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Һ��pH��7 | B�����Һ��c(B+)��c(A��) |
| C��a��b | D����������Գ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��������ƽ�������롢���ӣ� | B���ζ��� | C��100mL����Ͳ | D��100mL������ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
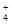 ����c��SO
����c��SO ��֮���� ( )
��֮���� ( )| A������2��1 | B������ 2��1 | C��С��2��1 | D�����ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
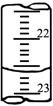
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com