
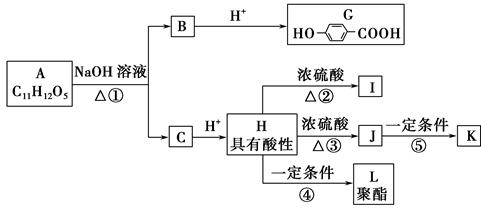
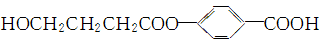
 H2O��
H2O��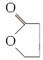
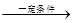
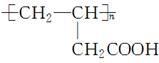
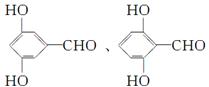
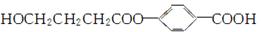 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
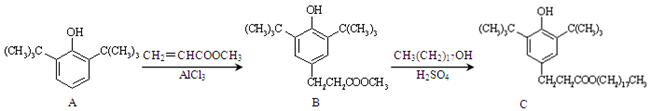
| A������A������̼ԭ���п���λ��ͬһƽ�� |
| B������B���ܷ���ȡ����Ӧ |
| C��������KMnO4��Һ����������C���Ƿ���CH3(CH2)17OH |
| D��1mol������C��NaOH��Һ��Ӧ����������2mol NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
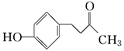 ����һ�ִ�ֲ�︲��������ȡ�Ľ�Ϊ��ȫ�����ϡ�һ����ʯ�ͻ�����ƷA�ͱ�ϩΪԭ�ϵĺϳ�·�����£�
����һ�ִ�ֲ�︲��������ȡ�Ľ�Ϊ��ȫ�����ϡ�һ����ʯ�ͻ�����ƷA�ͱ�ϩΪԭ�ϵĺϳ�·�����£�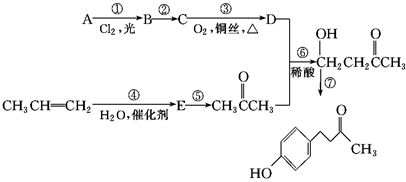
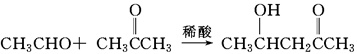
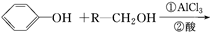
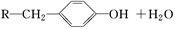
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
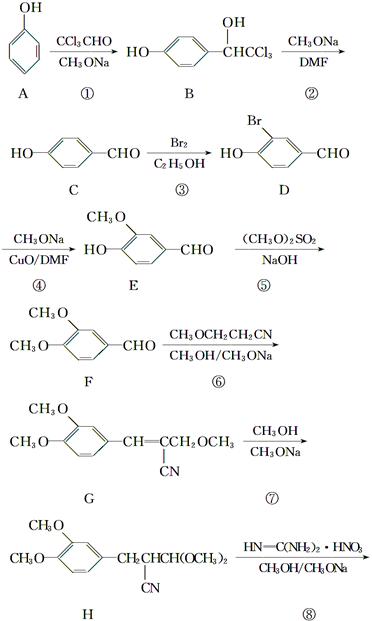
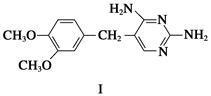
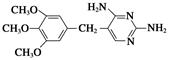 �ĸ����Ϊ��ʹ�ò���ĺ���������ͣ���Ҫ�ϳ�·���е�________����Ӧ�Ĺ������Ż���
�ĸ����Ϊ��ʹ�ò���ĺ���������ͣ���Ҫ�ϳ�·���е�________����Ӧ�Ĺ������Ż���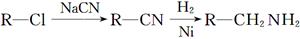 ��������
��������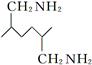 �Ǻϳ�Ⱦ�ϵ��м��壬��д����������Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�Ǻϳ�Ⱦ�ϵ��м��壬��д����������Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£� CH2=CH2
CH2=CH2 ?CH2��CH2?
?CH2��CH2?�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
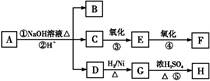
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
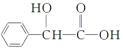 )������ͼ����ش��������⣺
)������ͼ����ش��������⣺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ԭ���ޱ�ըΣ�� | B��û�и����ԭ�������ʸ� |
| C��ԭ�϶��������� | D�����豸��ʴ�Խ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ���ͱ�ͪ��
���ͱ�ͪ�� ���Ʊ�������
���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�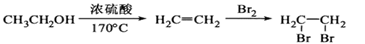
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������



 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�  ����ĵ�һ���ĺϳ�·�ߺ����һ���Ļ�ѧ����ʽ�����Լ���ѡ����
����ĵ�һ���ĺϳ�·�ߺ����һ���Ļ�ѧ����ʽ�����Լ���ѡ�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com