
��þ�����Ļ����0.1 mol����100 mL 2 mol/L��H2S04��Һ�У�Ȼ���ٵμ�1 mol/L��NaOH��Һ����ش�
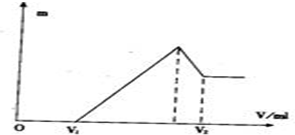
��1�����ڵμ�NaOH��Һ�Ĺ����У��������������NaOH��Һ�����V�仯����ͼ��ʾ����V1=160 mLʱ���������ĩ�У�n(Mg) =�� ��mol��V2=�� ��mL��
��2�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2+��A13 +�պó�����ȫ�������NaOH��Һ�����Ϊ����
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ�����Ļ����0.1mol ����100mL 2mol/LH2SO4��Һ�У�Ȼ���ڵõ�����Һ�еμ�1mol/LNaOH ��Һ����ش�
��1�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2+��Al3+�պ���ȫ�����������NaOH��Һ�������V(NaOH)=________mL��
��2�����ڵμ�NaOH��Һ�����У����������������NaOH��Һ������仯����ͼ��ʾ����V1=160mLʱ���������ĩ��þ�����ʵ�����V2���������Ҫ��д��������̣�

(3)���������Ϊ0.1mol ������Mg�����ʵ�������Ϊa����100mL 2mol/L��H2SO4�ܽ�˻������ټ���450mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3���������������a��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ�����Ļ����0.1mol ����100mL 2mol/LH2SO4��Һ�У�Ȼ���ڵõ�����Һ�еμ�1mol/LNaOH ��Һ����ش�
��1�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2+��Al3+�պ���ȫ�����������NaOH��Һ�������V(NaOH)=________mL��
��2�����ڵμ�NaOH��Һ�����У����������������NaOH��Һ������仯����ͼ��ʾ����V1=160mLʱ���������ĩ��þ�����ʵ�����V2���������Ҫ��д��������̣�
(3)���������Ϊ0.1mol ������Mg�����ʵ�������Ϊa����100mL 2mol/L��H2SO4�ܽ�˻������ټ���450mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3���������������a��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭��һ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��þ�����Ļ����0.1mol ����100mL 2mol/LH2SO4��Һ�У�Ȼ���ڵõ�����Һ�еμ�1mol/LNaOH ��Һ����ش�
��1�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2+��Al3+�պ���ȫ�����������NaOH��Һ�������V(NaOH)=________mL��
��2�����ڵμ�NaOH��Һ�����У����������������NaOH��Һ������仯����ͼ��ʾ����V1=160mLʱ���������ĩ��þ�����ʵ�����V2���������Ҫ��д��������̣�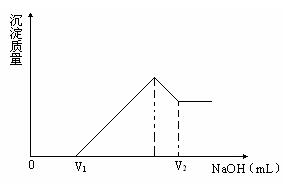
(3)���������Ϊ0.1mol ������Mg�����ʵ�������Ϊa����100mL 2mol/L��H2SO4�ܽ�˻������ټ���450mL 1mol/L��NaOH��Һ�����ó�������Al(OH)3���������������a��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��һ��ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�������
��7�֣���þ�����Ļ����0.1 mol����100 mL 2 mol/L��H2SO4��Һ�У�Ȼ��μ�1 mol/L��NaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У�����������m�������NaOH��Һ�������V���仯��ͼ��ʾ����ش�
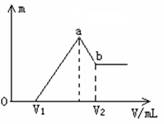
��1������V1=160ʱ���������ĩ��
n(Mg)= mol��V2= mL��
��2�����ڵμ�NaOH��Һ�Ĺ����У���ʹMg2+��Al3+�պó�����ȫ�������NaOH��Һ�����ӦΪ mL��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com