
| ѡ�� | ʵ����� | ���� | ���� |
| A�� | ��ij��Һ�м������� | ������ɫ���� | ��Һ��һ������CO32? |
| B�� | ����Fe(OH)2¶���ڿ�����һ��ʱ�� | ��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ | ˵��Fe(OH)2�ױ�������Fe(OH)3 |
| C�� | ��CuSO4��Һ�м���KI��Һ���ټ��뱽���� | �а�ɫ�����������������ɫ | ��ɫ��������ΪCuI |
| D�� | ��ij��ɫ��Һ�еμ������ữ��BaCl2��Һ | ������ɫ���� | ��Һ��һ������SO42- |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���ᴿ������ | ѡ�õ��Լ� | �������� |
| A | NaOH��Na2CO3�� | ���� | �� |
| B | CO2��CO�� | ���� | ���� |
| C | Fe��Zn�� | FeSO4��Һ | ���� |
| D | NaCl��Na2SO4�� | BaCl2��Һ | ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ü��ȵķ�����ȥ����̼���ƹ����е�̼�����ƹ��� |
| B����ȥCO��������O2���ɽ��������ͨ�����ȵ�Cu�� |
| C����ij��Һ�м������ᣬ����ʹ����ʯ��ˮ����ǵ����壬����Һ��һ������CO32- |
| D����ij��Һ�м��������ữ��BaCl2��Һ�а�ɫ����������Һ��һ������SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢݢܢ٢� | B���ܢ٢ڢݢ� |
| C���ܢڢݢ٢� | D���٢ܢڢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��ij��Һ �а�ɫ������˵��ԭ��Һ����Cl�� �а�ɫ������˵��ԭ��Һ����Cl�� |
B��ij��Һ �а�ɫ������˵��ԭ��Һ����SO42�� �а�ɫ������˵��ԭ��Һ����SO42�� |
C��ij��Һ ����ɫ������˵��ԭ��Һ����Cu2+ ����ɫ������˵��ԭ��Һ����Cu2+ |
D��ij��Һ ������ɫ���壬˵��ԭ��Һ����CO32�� ������ɫ���壬˵��ԭ��Һ����CO32�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢܢ� | B���ڢݢ٢ۢ� | C���٢ۢݢڢ� | D���ڢ٢ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
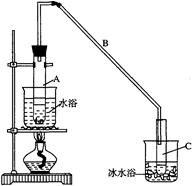

| | �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
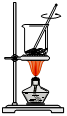 |  |  |  |
| A���������ճɻ� B�����˵ú�I����Һ C���ų���ı���Һ D������Ⲣ���ձ� | |||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com