
��15�֣��±��dz�ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
��ش��������⣺
(1)��������d����Ԫ���� �����ţ���
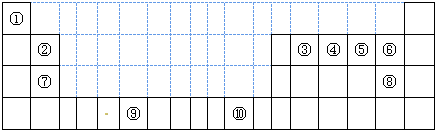
(2)��ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ��ͼ�Тڡ��ܡ���ֱ�λ�� ���������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ ���ö�Ӧ��Ԫ�ط��ű�ʾ����
���������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ ���ö�Ӧ��Ԫ�ط��ű�ʾ����
(3)Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ �ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����
�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����  ��
��
a�������к������ b�����ڷǼ��Է���
c������4���Ҽ���1���м� d�����⻯������У���ԭ�Ӳ���sp2�ӻ�
(4)ijԪ�ص���Χ�����Ų�ʽΪnsnnpn+1����Ԫ�ؿ�����γ������εķ���X�� X�ڢ�����γɵķ���Y�е��ܽ�Ⱥܴ�����Ҫԭ����
��
(5)��������Xͨ�뺬��Ԫ�آ����������Һ�У������� ����Ӧ�����ӷ���ʽ_ ��
(6) �ⵥ�ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��
����֪���ԭ�Ӱ뾶Ϊd��NA���������ӵ�������������ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ________���þ�����ܶ�Ϊ___ _____������ĸ��ʾ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��15�֣��±��dz�ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�

��ش��������⣺
(1)��������d����Ԫ���� �����ţ���
(2)��ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ ���ö�Ӧ��Ԫ�ط��ű�ʾ����

(3)Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ���� ��
a�������к������ b�����ڷǼ��Է���
c������4���Ҽ���1���м� d�����⻯������У���ԭ�Ӳ���sp2�ӻ�
(4)ijԪ�ص���Χ�����Ų�ʽΪnsnnpn+1����Ԫ�ؿ�����γ������εķ���X�� X�ڢ�����γɵķ���Y�е��ܽ�Ⱥܴ�����Ҫԭ����
��
(5)��������Xͨ�뺬��Ԫ�آ����������Һ�У������� ����Ӧ�����ӷ���ʽ_ ��
(6) �ⵥ�ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��

����֪���ԭ�Ӱ뾶Ϊd��NA���������ӵ�������������ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ________���þ�����ܶ�Ϊ___ _____������ĸ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣��±��dz�ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
��ش��������⣺
(1)��������d����Ԫ���� �����ţ���
(2)��ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ ���ö�Ӧ��Ԫ�ط��ű�ʾ����
(3)Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ���� ��
a�������к������ b�����ڷǼ��Է���
c������4���Ҽ���1���м� d�����⻯������У���ԭ�Ӳ���sp2�ӻ�
(4)ijԪ�ص���Χ�����Ų�ʽΪnsnnpn+1����Ԫ�ؿ�����γ������εķ���X�� X�ڢ�����γɵķ���Y�е��ܽ�Ⱥܴ�����Ҫԭ����
��
(5)��������Xͨ�뺬��Ԫ�آ����������Һ�У������� ����Ӧ�����ӷ���ʽ_ ��
(6) �ⵥ�ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��
����֪���ԭ�Ӱ뾶Ϊd��NA���������ӵ�������������ԭ������ΪM����ش𣺾����Т�ԭ�ӵ���λ��Ϊ________���þ�����ܶ�Ϊ___ _____������ĸ��ʾ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com