
��֪��O2(g)= O2+(g)+e�� H1= +1175.7 kJ��mol-1
PtF6��(g)=PtF6(g)+e�� H2= +771.1 kJ��mol-1
O2+(g)+PtF6��(g)=O2PtF6(S) H3= -482.2 kJ��mol-1
��ӦO2(g)+PtF6(g)= O2PtF6 (s)��H�ǣ� ��
A��77.6 kJ B��-77.6 kJ��mol-1 C��+77.6kJ��mol-1 D��-886.8kJ��mol-1
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��1���ϳɰ���ӦN2(g)��3H2(g)![]() 2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô��� ��Ӧ�ġ�H�����������С�����ı䡱����
2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô��� ��Ӧ�ġ�H�����������С�����ı䡱����
��2����֪��O2(g) = O2+(g)+e�� ![]() H1=1175.7 kJ��mol-1
H1=1175.7 kJ��mol-1
PtF6(g)+e��=PtF6��(g) ![]() H2=-771.1 kJ��mol-1
H2=-771.1 kJ��mol-1
O2PtF6(S)=O2+(g)+PtF6��(g) ![]() H3=482.2 kJ��mol-1
H3=482.2 kJ��mol-1
��ӦO2(g)+PtF6(g) = O2+PtF6��(s)��![]() H=_____________ kJ��mol-1��
H=_____________ kJ��mol-1��
��3����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ�������� �������ѧʽ�������ɸó��������ӷ���ʽΪ ����֪25��ʱKsp[Mg(OH)2]=1.8��10-11��KsP[Cu(OH)2]=2.2��10-20��
��4����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧʱ��Һ��c(NH4*)=c(Cl-)������Һ�� �ԣ���ᡱ��������С������ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������������������ѧ�߶����п��Ի�ѧ�Ծ����������� ���ͣ������
��10�֣����û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��1���ϳɰ���ӦN2(g)��3H2(g) 2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô�����Ӧ�ġ�H �����������С�����ı䡱����
2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ�� �ƶ�������������ҡ���������ʹ�ô�����Ӧ�ġ�H �����������С�����ı䡱����
��2����֪��O2(g) = O2+(g)+e�� 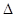 H1=��1175.7 kJ��mol-1
H1=��1175.7 kJ��mol-1
PtF6(g)+e��=PtF6��(g) 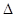 H2=��771.1 kJ��mol-1
H2=��771.1 kJ��mol-1
O2PtF6(S)=O2+(g)+PtF6��(g) 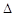 H3=��482.2 kJ��mol-1
H3=��482.2 kJ��mol-1
��ӦO2(g)+PtF6(g) = O2+PtF6��(s)�� 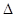 H="_____________" kJ��mol-1��
H="_____________" kJ��mol-1��
��3����֪��2NO2��g��  N2O4��g�� ��H=-57.2kJ��mol-1��һ���¶��£�һ��������ܱ������г���NO2���з�Ӧ��2NO2��g��
N2O4��g�� ��H=-57.2kJ��mol-1��һ���¶��£�һ��������ܱ������г���NO2���з�Ӧ��2NO2��g�� N2O4��g���ﵽƽ�⡣д���÷�Ӧ��ƽ�ⳣ������ʽ������ ���������¶ȣ��÷�Ӧ��ƽ�ⳣ��Kֵ���� (��������С��)��
N2O4��g���ﵽƽ�⡣д���÷�Ӧ��ƽ�ⳣ������ʽ������ ���������¶ȣ��÷�Ӧ��ƽ�ⳣ��Kֵ���� (��������С��)�� ��������������ʱ�����д�ʩ�����NO2ת���ʵ��� ������ĸ��ţ�
��������������ʱ�����д�ʩ�����NO2ת���ʵ��� ������ĸ��ţ�
| A����СNO2��Ũ�� | B�������¶� | C������NO2�����ʵ��� | D�������¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰ����Һ�е����ӷ�Ӧ��ר���ۺϲ��ԣ��ս̰棩 ���ͣ������
(8��)(2009��ɽ����)���û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
(1)�ϳɰ���ӦN2(g)��3H2(g) ===2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��________�ƶ�(��������ҡ�����)��ʹ�ô���________��Ӧ�Ħ�H(�������С�����ı䡱)��
(2)��֪��O2(g)===O2��(g)��e��
��H1��1175.7 kJ��mol��1��
PtF6(g)��e��===PtF6��(g)
��H2����771.1 kJ��mol��1��
O2��PtF6��(s)===O2��(g)��PtF6��(g)
��H3��482.2 kJ��mol��1��
��ӦO2(g)��PtF6(g)===O2��PtF6��(s)�Ħ�H��________kJ��mol��1��
(3)��25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��MgCl2��CuCl2�����Һ����μ��백ˮ��������________����(�ѧʽ)�����ɸó��������ӷ���ʽΪ________����֪25��ʱKsp[Mg(OH)2]��1.8��10��11��Ksp[Cu(OH)2]��2.2��10��20��
(4)��25���£���a mol��L��1�İ�ˮ��0.01 mol��L��1������������ϣ���Ӧƽ��ʱ��Һ��c(NH4��)��c(Cl��)������Һ��________��(��ᡱ������С�)���ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�����и����ڶ���������⻯ѧ�Ծ� ���ͣ�ѡ����
��֪��O2(g)
= O2+(g)+e��
 H1= +1175.7 kJ��mol-1
H1= +1175.7 kJ��mol-1
PtF6��(g)
=PtF6(g)+e��  H2= +771.1 kJ��mol-1
H2= +771.1 kJ��mol-1
O2+(g)+PtF6��(g)
=O2PtF6(S)  H3= -482.2 kJ��mol-1
H3= -482.2 kJ��mol-1
��ӦO2(g)+PtF6(g)
= O2PtF6 (s)�� H�ǣ� ��
H�ǣ� ��
A��77.6 kJ B��-77.6 kJ��mol-1 C��+77.6kJ��mol-1 D��-886.8kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�߶��¶�ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��9�֣����û�ѧ��Ӧԭ���о��������ȵ��ʼ��仯����ķ�Ӧ����Ҫ���塣
��1���ϳɰ���ӦN2(g)��3H2(g) 2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��
�ƶ�������������ҡ���������ʹ�ô�����Ӧ�ġ�H
�����������С�����ı䡱����
2NH3(g)�����ں��¡���ѹ��������ƽ����ϵ��ͨ�������ƽ��
�ƶ�������������ҡ���������ʹ�ô�����Ӧ�ġ�H
�����������С�����ı䡱����
��2����֪��O2(g)
= O2+(g)+e��
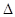 H1=��1175.7 kJ��mol-1
H1=��1175.7 kJ��mol-1
PtF6(g)+e��=PtF6��(g)
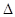 H2=��771.1 kJ��mol-1
H2=��771.1 kJ��mol-1
O2PtF6(S)=O2+(g)+PtF6��(g)
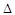 H3=��482.2 kJ��mol-1
H3=��482.2 kJ��mol-1
��ӦO2(g)+PtF6(g) = O2+PtF6��(s)�� 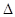 H=_____________ kJ��mol-1��
H=_____________ kJ��mol-1��
��3����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��MgCl2��CuCl2�����Һ����μ��백ˮ�������� �������ѧʽ�������ɸó��������ӷ���ʽΪ ��
��֪25��ʱKsp[Mg(OH)2]=1.8��10-11��KsP[Cu(OH)2]=2.2��10-20��
��4����25���£���a mol��L-1�İ�ˮ��0.01 mol��L-1������������ϣ���Ӧʱ��Һ��
c(NH4��)=c(Cl��)������Һ�� �ԣ���ᡱ��������С������ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb= ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com