
| ʵ���� | ��ˮ���ʵ���Ũ��/ ��mol•L-1�� | �������ʵ���Ũ��/ ��mol•L-1�� | �����ҺpH |
| �� | 0.1 | 0.1 | pH=5 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
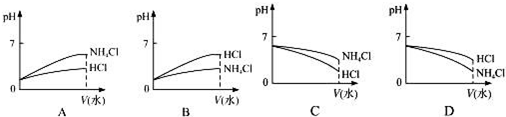
���� ��1������Һ���Ȼ����Һ�������ˮ����ʾ���ԣ���Һ�е������Ӿ���ˮ����ģ�
������ҺPH=7����Һ��ʾ���ԣ�c=0.2ʱ����Һ��ʾ���ԣ�Ҫ��ʾ���ԣ���ˮ�����Щ��
���з�Ӧ����������Ȼ�狀Ͱ�ˮ����PH��7����ˮ�ĵ���̶ȴ�������ӵ�ˮ�⣬��Һ�������Ũ�ȴ��ڰ�ˮŨ�ȣ�
��2��pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı������Ȼ����Һ��pH�仯��С���Ȼ�����Һ��pH�仯�ϴݴ��жϣ�
��3��A�����ݵ���غ㣬����Һ�����ԣ�c��H+��=c��OH-��������c ��C1-��=c��NH4+������ʱ��ˮӦ����������
B���������Թ���ʱ��c��Cl-����c��NH4+��=c��H+����c��OH-����
C����ϵΪNH4Cl��Һ��NH3��H2O����ˮ�����϶�ʱ����Һ�ʼ��ԣ�c��NH4+����c��OH-����c��Cl-����c��H+����
D��������1��1�ĵ��������ӣ������ӱ���ˮ�к�һ���֣�����c��H+�������ܴ��� c��Cl-����
��� �⣺��1���ӵڢ�������������������Ȼ����Һ�������ˮ�⣬��Һ��ʾ���ԣ���Һ�е���������ˮ����ģ�������ˮ�������c��H+��=1��10-5mol•L-1��
�ӵڢ������������pH=7����Һ��ʾ���ԣ���c=0.2�������Ȼ����Һ����ʾ���ԣ��ʰ�ˮ��Ũ���Դ�Щ����c��0.2��
�ӵڢ������������֪��pH��7����ˮ�ĵ���̶ȴ���ˮ��̶ȣ��������Ũ�ȴ��ڰ�ˮŨ�ȣ�
�ʴ�Ϊ��1��10-5����������
��2��pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı������Ȼ����Һ��笠�����ˮ�����Һ��ʾ���ԣ�ϡ�ͺ�笠�����ˮ��̶�������Һ�������ӵ����ʵ��������Ȼ���Ϊǿ����ʣ�ϡ������������Ũ�ȼ�С������ϡ�ͺ�����Һ��pH���������Ȼ�����Һ��pH�仯�ϴ�ͨ���۲�ͼ���֪����ȷ��ѡ��ΪB��
�ʴ�Ϊ��B��
��2��A�����ݵ���غ㣬����Һ�����ԣ�c��H+��=c��OH-��������c ��C1-��=c��NH4+������ʱ��ˮӦ����������c��C1-��=c��NH4+����c��H+��=c��OH-������A��ȷ��
B���������Թ���ʱ��c��Cl-����c��NH4+��=c��H+����c��OH-������B��ȷ��
C����ϵΪNH4Cl��Һ��NH3��H2O����ˮ�����϶�ʱ����Һ�ʼ��ԣ�c��NH4+����c��OH-����c��Cl-����c��H+������C��ȷ��
D��������1��1�ĵ��������ӣ������ӱ���ˮ�к�һ���֣�����c��H+�������ܴ��� c��Cl-������D����
�ʴ�Ϊ��ABC��
���� ������Ҫ��������Ũ�ȴ�С�Ƚϡ�������ʵ���ƽ��Ӱ�����ء���Һ����Ե�֪ʶ�����Ը�����ѧ֪ʶ��ɣ���Ŀ�Ѷ��еȣ�


 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ȶ������Һ�� | B�� | �����ܹ�����ȡ����Ӧ | ||
| C�� | �����е�̼ԭ�Ӳ���һ��ֱ���� | D�� | ��ʯ�ͷ����һ�ֲ�Ʒ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��3���ȱ��� | B�� | 1��1���ȱ��� | C�� | 1��2���ȱ��� | D�� | 2��2���ȱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al��Si��P | B�� | I��Br��Cl | C�� | O��S��Na | D�� | Na��Mg��Ba |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȡ25.0gCuSO4•5H2O����1Lˮ�� | |
| B�� | ȡ16.0gCuSO4•5H2O����ˮ����ϡ����1L | |
| C�� | ����������ʧȥ�ᾧˮ����ȡ��ˮ����ͭ16.0g����1Lˮ | |
| D�� | ȡ25.0gCuSO4•5H2O����ˮ����ϡ�����1L��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2��D2��T2����Ϊͬ�������� | B�� | 35Cl��37Cl ����Ϊͬλ�� | ||
| C�� |  �� ��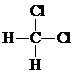 ����Ϊͬ���칹�� ����Ϊͬ���칹�� | D�� | C2H4��C4H8һ���ܻ���Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2 | B�� | O2 | C�� | SO2 | D�� | NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
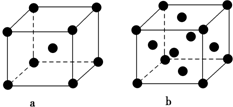
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ•mol-1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com