
��������������±���ʾ��
| ���� | �۵�/�� | �е�/�� | ����ܶ�(20��) | ���� | ˮ���� |
| �� | 5.5 | 80.1 | 0.8794 | �� | ������ˮ |
| ���� | ��41.59 | 83 | 1.503 | | ������ˮ |
| ������ | 5.7 | 210.9 | 1.205 | �� | ������ˮ |
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��1�� | ��2�� | ��3�� |
| S�����ʣ� | SO2��X��Na2SO3��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������±���ʾ��
| ���� | �۵�/�� | �е�/�� | ����ܶ�(20��) | ���� | ˮ���� |
| �� | 5.5 | 80.1 | 0.8794 | �� | ������ˮ |
| ���� | ��41.59 | 83 | 1.503 |
| ������ˮ |
| ������ | 5.7 | 210.9 | 1.205 | �� | ������ˮ |
�ɹ�ѡ���ʵ����Ʒ��
���������ձ������Թܡ�����̨(����Ȧ)��ʯ�������ƾ��ơ���Ƥ���������ܡ��¶ȼơ���Һ©���ȡ�
��ҩƷ��Ũ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢ����5%NaOH��Һ������ˮ�ȡ�
��ο�����ʵ�����˼·�����۲��ش��й����⡣
(2)ѡ����ʵ�ʵ������������
�ٲ��õļ��ȷ�ʽ��
________________________________________________________________________��
���ŵ���
________________________________________________________________________��
�ڷ�Ӧ���Ϊ________������________����Ӧ����
�۷�Ӧ�������������ӷ������ʣ�Ϊ��ֹ��ʧ����________�������________���á�
����________������________���¶ȡ�
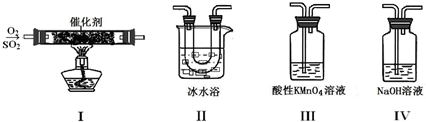
���뻭��ʵ��װ�ü�ͼ��
(2)���ź�����ʵ�鲽��
�ٰ�װ��ͼ��װ��ʵ��������
���ڴ��Թ���ȼ���1.5 mLŨ�����2mLŨ���ᣬҡ�ȣ���ȴ��50��60�档
������������1 mL������������ҡ����ʹ֮��Ͼ��ȣ����õ�������
�ܷ���50��60���ˮԡ�м���10 min��
(3)��Ʒ�ᴿ
̽�����ۣ���Ũ�����Ũ����Ļ��˳���ܷ�ߵ���Ϊʲô��
________________________________________________________________________��
��Ũ�����������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�Ͼ�ѧ�����ר��ѧУ��һ4���¿���ѧ�Ծ����������� ���ͣ�ʵ����
ijС��ͬѧ���о�SO2�����ʡ�
(1)����صĺ������ʷ�Ϊ���±���ʾ3�飬��2��������X�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S(����) | SO2��X��Na2SO3��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰ����ʶ�л���Ľṹ����ࡢ����������ר���ۺϲ��� ���ͣ�ʵ����
��������������±���ʾ��
|
���� |
�۵�/�� |
�е�/�� |
����ܶ�(20��) |
���� |
ˮ���� |
|
�� |
5.5 |
80.1 |
0.8794 |
�� |
������ˮ |
|
���� |
��41.59 |
83 |
1.503 |
|
������ˮ |
|
������ |
5.7 |
210.9 |
1.205 |
�� |
������ˮ |
�ɹ�ѡ���ʵ����Ʒ��
���������ձ������Թܡ�����̨(����Ȧ)��ʯ�������ƾ��ơ���Ƥ���������ܡ��¶ȼơ���Һ©���ȡ�
��ҩƷ��Ũ���ᡢϡ���ᡢŨ���ᡢϡ���ᡢ����5%NaOH��Һ������ˮ�ȡ�
��ο�����ʵ�����˼·�����۲��ش��й����⡣
(2)ѡ����ʵ�ʵ������������
�ٲ��õļ��ȷ�ʽ��
________________________________________________________________________��
���ŵ���
________________________________________________________________________��
�ڷ�Ӧ���Ϊ________������________����Ӧ����
�۷�Ӧ�������������ӷ������ʣ�Ϊ��ֹ��ʧ����________�������________���á�
����________������________���¶ȡ�
���뻭��ʵ��װ�ü�ͼ��
(2)���ź�����ʵ�鲽��
�ٰ�װ��ͼ��װ��ʵ��������
���ڴ��Թ���ȼ���1.5 mLŨ�����2 mLŨ���ᣬҡ�ȣ���ȴ��50��60�档
������������1 mL������������ҡ����ʹ֮��Ͼ��ȣ����õ�������
�ܷ���50��60���ˮԡ�м���10 min��
(3)��Ʒ�ᴿ
̽�����ۣ���Ũ�����Ũ����Ļ��˳���ܷ�ߵ���Ϊʲô��
________________________________________________________________________��
��Ũ�����������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com