
��06�꽭�վ���(8��)±�ػ�������ָ��ͬ±��ԭ��֮���Թ��ۼ�����γɵĻ����XX����±�ػ�������±�ص��ʽṹ���ơ�����������Իش��������⣺
��±�ػ�����BrCl�ܷ������з�Ӧ
H2O��BrCl===HBrO��HCl
 KBr��BrCl===KCl��Br2
KBr��BrCl===KCl��Br2
��д��KI��IBr��Ӧ�Ļ�ѧ����ʽ______________��
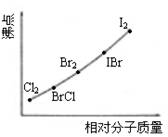
��д������(C6H6)��ICl����ȡ����Ӧ����һ±����Ļ�ѧ����ʽ____________________________������ͼ�Dz���±�ص��ʺ�XX����±�ػ�����ķе�������Է��������Ĺ�ϵͼ�����ǵķе�������Է�����������������ߣ���ԭ����______________��
�����Ʋ�ICl�ķе���������С��Χ______________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06�꽭�վ���ij��ѧ��ȤС�鰴�����з������С��ɺ��������Ʊ����������塱��ʵ�飺
����1��ȡһ����������������������NaOH��Һ����Ӧ��ȫ����ˡ�
����2���߽��������Һ�еμ�ϡ��������ҺpH��8��9�����á����ˡ�ϴ�ӡ�
����3��������2�еõ��Ĺ�������������ϡ���ᡣ
����4�����õ�����Һ����Ũ������ȴ���ᾧ�����ˡ����
��ش��������⣺
��1������ʵ���еĹ��˲�����Ҫ�������� �� �Ȳ���������
��2������1���˵�Ŀ���� ��
��3��������2����Һ��pH��8��9ʱ����������Ƿ���ȫ�ķ����� ��
��4������2����Һ��pH���ѿ��ƣ��ɸ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Իش��������⣺
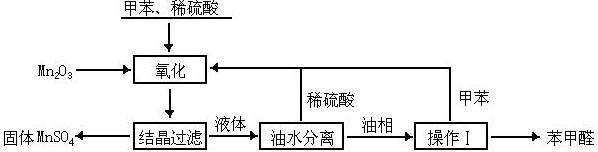
��Mn2O3�����ױ��ķ�Ӧ��Ҫ���Ͻ��裬�����������______________��
�Ƽױ���������õ��Ļ����ͨ���ᾧ�����˽��з��롣�ù������轫�������ȴ����Ŀ����______________��
��ʵ������У���ѭ��ʹ�õ����ʷֱ�Ϊ_______��_______��
��ʵ���з���ױ��ͱ���ȩ���õIJ�������______________����ԭ����______________��
��ʵ���з��֣���Ӧʱ�䲻ͬ����ȩ�IJ���Ҳ��ͬ�����ݼ��±�����
��Ӧʱ��/h | 1 | 2 | 3 | 4 | 5 |
����ȩ����/% | 76.0 | 87.5 | 83.6 | 72.5 | 64.8 |
���ϱ���ȩ�Ľṹ����������Ӧʱ�����ʱ������ȩ�����½���ԭ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06�꽭�վ���(10��)����ȩ��ҽҩ��Ⱦ�ϡ����ϵ���ҵ���Ź㷺��Ӧ�á�ʵ����ͨ����ͼ��ʾ�������ɼױ������Ʊ�����ȩ��

�Իش��������⣺
��Mn2O3�����ױ��ķ�Ӧ��Ҫ���Ͻ��裬�����������______________��
�Ƽױ���������õ��Ļ����ͨ���ᾧ�����˽��з��롣�ù������轫�������ȴ����Ŀ����______________��
��ʵ������У���ѭ��ʹ�õ����ʷֱ�Ϊ_______��_______��
��ʵ���з���ױ��ͱ���ȩ���õIJ�������______________����ԭ����______________��
��ʵ���з��֣���Ӧʱ�䲻ͬ����ȩ�IJ���Ҳ��ͬ�����ݼ��±�����
��Ӧʱ��/h | 1 | 2 | 3 | 4 | 5 |
����ȩ����/% | 76.0 | 87.5 | 83.6 | 72.5 | 64.8 |
���ϱ���ȩ�Ľṹ����������Ӧʱ�����ʱ������ȩ�����½���ԭ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06�꽭�վ���(8��)�Ȼ���ͭ��CuCl������Ҫ�Ļ���ԭ�ϡ����ұ��涨�ϸ��CuCl��Ʒ����Ҫ����ָ��ΪCuCl��������������96.50%����ҵ�ϳ�ͨ�����з�Ӧ�Ʊ�CuCl
2CuSO4��Na2SO3��2NaCl��Na2CO3===2CuCl����3Na2SO4��CO2��
��CuCl�Ʊ���������Ҫ������������Ϊ20.0%��CuSO4��Һ���Լ������ø���Һ�����CuSO4?5H2O��H2O������֮�ȡ�
��ȷ��ȡ�����õ�0.2500g CuCl��Ʒ����һ������0.5mol?L��1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol?L��1��Ce(SO4)2��Һ�ζ����յ�,����24.60mLCe(SO4)2��Һ���йط���ѧ��ӦΪ
Fe3+��CuCl===Fe2+��Cu2+��Cl-
Ce4+��Fe2+===Fe3+��Ce3+
ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com