
��14�֣���֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����Ӧ����ʽΪ
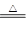 2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��A�мӵ����Ȼ�狀��������ƹ��壬C�еĹ���������ͭ���ش��������⣺
2NH3��3CuO N2��3H2O��3Cu����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��A�мӵ����Ȼ�狀��������ƹ��壬C�еĹ���������ͭ���ش��������⣺
��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
���鰱��ͨ�����õķ�����________ _����������__ ________��
��2��B���������� ���������� ��
��3��ʵ��ʱC�й۲쵽�������� ���÷�Ӧ�а���������_______������������ԭ������
��4��ʵ��ʱ��D���ռ�����Һ̬������ ��E���ռ�����������__________��
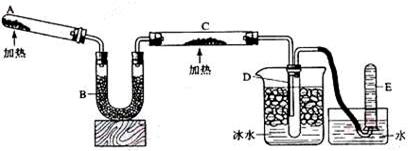
��5����Ҫ���鷴Ӧ���ɵ�ˮ���ɽ��Թ�D��װ��ˮ���ձ����ָij���ͼװ�ã�U�ι�X��װ��____________��������________________�������Y��װ�м�ʯ�ң�������_______________________________________��
��1��2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O��2�֣�
CaCl2+2NH3��+2H2O��2�֣�
��ʪ��ĺ�ɫʯ����ֽ���飬��ֽ������2�֣�
��2����ʯ�ң���ȥ�����е�ˮ������2�֣�
��3����ɫ������ɺ�ɫ����ԭ����2�֣� ��4����ˮ��������2�֣�
��5��������ͭ��������2�֣�����ֹE�е�ˮ������������X��Ӱ��ˮ�ļ��飨2�֣�
����������1������A�е��Լ����жϣ�A��ʵ������ȡ�����ġ���������ˮ�Լ��ԣ��ݴ˿���������ʪ��ĺ�ɫʯ����ֽ���飬�����DZ���ɫ��
��2����Ϊ������ͭ��Ӧ�İ���Ӧ�Ǹ���ģ�����B��ԭ�����ﰱ���ģ�����ѡ���ʯ�ҡ�
��3�����ݷ���ʽ�����жϣ�����������ͭ�������ɵ�����ˮ������ͭ��ԭ���ɺ�ɫ��ͭ��
��4����������ˮ����ȴ����Һ̬ˮ�����ڰ�����������ȫ������������ʣ��İ�������ˮ���ɰ�ˮ�����ɵĵ���������ˮ�����ռ���E�С�
��5������ˮ�Ĵ���һ������ˮ����ͭ������ͭ��ˮ��������ɫ�ĵ��������ں���ĵ����Dz���ˮ�еģ�����Ϊ�˷�ֹ�����ˮ��������U�ιܸ���ˮ�ļ��飬��Ҫ����1��װ�м�ʯ�ҵĸ���ܡ�


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
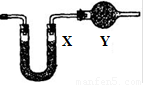
![]() ��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��
��֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��
![]()
![]()

![]()
![]()
![]()
![]() �ش��������⣺
�ش��������⣺
![]()
![]() ��1��A����������_____________��_____________________________.
��1��A����������_____________��_____________________________.
![]()
![]() ������Ӧ�Ļ�ѧ����ʽ��_______________��_______________________;
������Ӧ�Ļ�ѧ����ʽ��_______________��_______________________;
![]()
![]() ��2��B����������_____��________����������_________��_______________:
��2��B����������_____��________����������_________��_______________:
![]()
![]() ��3��ʵ��ʱ��C�й۲쵽��������_____________��_________________��
��3��ʵ��ʱ��C�й۲쵽��������_____________��_________________��
![]()
![]() ������Ӧ�Ļ�ѧ����ʽ��_________________��____________________;
������Ӧ�Ļ�ѧ����ʽ��_________________��____________________;
![]()
![]() (4) ʵ��ʱ��D�й۲쵽��������________________��__________________��
(4) ʵ��ʱ��D�й۲쵽��������________________��__________________��
![]()
![]() D���ռ�����������_______��_______,��������ʵķ�����������_________��_____________.
D���ռ�����������_______��_______,��������ʵķ�����������_________��_____________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����и߸�������ѧ��11�·��¿���ѧ�Ծ� ���ͣ�ʵ����
��12�֣�����֪�����������ȵ�����ͭ��Ӧ�õ������ͽ���ͭ����ʾ��ͼ�е�װ�ÿ���ʵ�ָ÷�Ӧ��

�ش��������⣺
��1��A�м���Ĺ�̬������� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��2��B���������� ���������� ��
��3��ʵ��ʱ�ڹ۲쵽C�е������� ���������������������� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��4��ʵ��ʱ��D�й۲쵽�������� ��D���ռ����������� ������������е�ijһ�����ʵķ����������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com