
��15�֣���֪AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1:1���м����C��������һ��Ҳ�ܸ����Ƶ�Cu(OH)2������Ӧ����ש��ɫ������CҲ�ܷ���������Ӧ��E����ζ��FΪһ�־ۺ������Ӧ����δд������
��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�__________________
��
��2��A����B�ķ�Ӧ����Ϊ_______________��C�Ľṹ��ʽΪ��______________________
B��D�й����ŵ����Ʒֱ���_____________��______________��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ��� ��
��4����Ӧ�ܵĻ�ѧ����ʽΪ�� _________
��Ӧ���ͣ� ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ��� ����ط������������ __�������ƣ���
��6����Ӧ�ݵĻ�ѧ����ʽΪ��____________________________________________________
��Ӧ���ͣ� ��
��15�֣���1��������ֱ�ͨ����������Һ������ˮ������ʹ��Һ��ɫ������ϩ����һΪ���飨���߽�����ֱ��ȼ���۲������ɫ�����������������̵�����ϩ������ɫ���Ǽ��飩������������Ҳ���֣���2�֣�
��2���ӳɷ�Ӧ��CH3CHO���ǻ����Ȼ�����1�֣���3������KMnO4��K2Cr2O7��Һ��1�֣�
��4����CH3COOH+ CH3CH2OHCH3COOCH2CH3 + H2O��2�֣� ������Ӧ��ȡ����Ӧ ��1�֣� ��5�� ����Na2CO3��Һ ��Һ ��ÿ�ո�1�֣�
��6��(2��) �Ӿ۷�Ӧ��1�֣�
����:



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꺣��ʡ�λ���ѧ��һ��ѧ�ڽ�ѧ������⻯ѧ��⣨���������� ���ͣ������
��15�֣���֪AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1:1���м����C��������һ��Ҳ�ܸ����Ƶ�Cu(OH)2������Ӧ����ש��ɫ������CҲ�ܷ���������Ӧ��E����ζ��FΪһ�־ۺ������Ӧ����δд������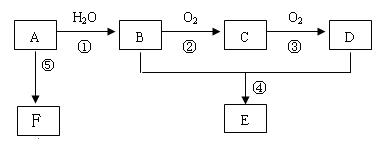
��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�__________________
��
��2��A����B�ķ�Ӧ����Ϊ_______________��C�Ľṹ��ʽΪ��______________________
B��D�й����ŵ����Ʒֱ���_____________��______________��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ� �� ��
�� ��
��4����Ӧ�ܵĻ�ѧ����ʽ Ϊ��
��  _________
_________
��Ӧ���ͣ� ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ��� ����ط������������
����ط������������  __�������ƣ���
__�������ƣ���
��6����Ӧ�ݵĻ�ѧ����ʽΪ��____________________________________________________
��Ӧ���ͣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�캣��ʡ��һ��ѧ�ڽ�ѧ������⻯ѧ��⣨���������� ���ͣ������
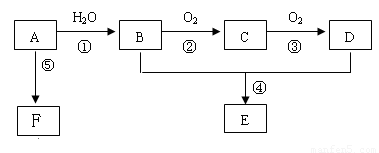
��15�֣���֪AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1:1���м����C��������һ��Ҳ�ܸ����Ƶ�Cu(OH)2������Ӧ����ש��ɫ������CҲ�ܷ���������Ӧ��E����ζ��FΪһ�־ۺ������Ӧ����δд������
��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�__________________
��
��2��A����B�ķ�Ӧ����Ϊ_______________��C�Ľṹ��ʽΪ��______________________
B��D�й����ŵ����Ʒֱ���_____________��______________��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ��� ��
��4����Ӧ�ܵĻ�ѧ����ʽΪ�� _________
��Ӧ���ͣ� ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ��� ����ط������������ __�������ƣ���
��6����Ӧ�ݵĻ�ѧ����ʽΪ��____________________________________________________
��Ӧ���ͣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
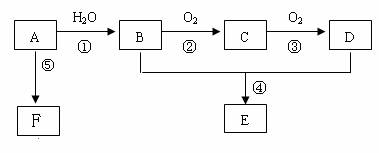
��֪AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1:1���м����C��������һ��Ҳ�ܸ����Ƶ�Cu(OH)2������Ӧ����ש��ɫ������CҲ�ܷ���������Ӧ��E����ζ��FΪһ�־ۺ������Ӧ����δд������
��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�__________________
��
��2��A����B�ķ�Ӧ����Ϊ_______________��C�Ľṹ��ʽΪ��______________________
B��D�й����ŵ����Ʒֱ���_____________��______________��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ��� ��
��4����Ӧ�ܵĻ�ѧ����ʽΪ�� _________
��Ӧ���ͣ� ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ��� ����ط������������ __�������ƣ���
��6����Ӧ�ݵĻ�ѧ����ʽΪ��____________________________________________________
��Ӧ���ͣ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com