
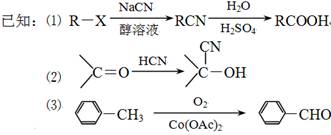

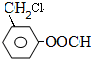
�������·����ϳɸ�Ч���Ͷ�ũҩɱ�����( ) ��
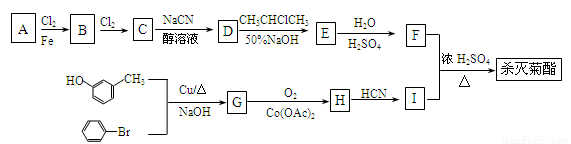
��1��F����ʽΪ_____________________���ϳ�G�ķ�Ӧ������______________��
��2���й�A������˵����ȷ����__________(�����)��
a��A�DZ������ͬϵ��
b��A�ĺ˴Ź���������5����
c��ȼ�յ����ʵ���A�ͻ��������������������
d��A������ԭ�ӿ��ܴ���ͬһƽ����
e��A����ʹ����KMnO4��Һ��ɫ
��3��д����ӦB��C�Ļ�ѧ����ʽ��ע����Ӧ��������_________________________��
��4��д��C����������������ˮ��Һ�����·�����ַ�Ӧ�Ļ�ѧ����ʽ��__________________
��5��D����������·���ˮ������J��D  J
J
д����������Ҫ���J������ͬ���칹��Ľṹ��ʽ(���Բ�����)��
________��________��________��_________��__________��
�ٱ�����������λ�ڼ�λ��ȡ������
����ˮ��������л��
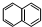
��6����-������X��I��Ϊͬ���칹�壬��X����( )��һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ��
��
)��һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ��
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ����Һ |
| ||
| H2SO4 |


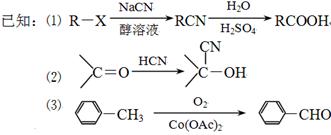
 ����
����
 +Cl2
+Cl2| ���� |
 +HCl
+HCl +Cl2
+Cl2| ���� |
 +HCl
+HCl +3NaOH��2NaCl+H2O+
+3NaOH��2NaCl+H2O+
 +3NaOH��2NaCl+H2O+
+3NaOH��2NaCl+H2O+
| ||
| H2O |







 ����һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ��
����һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������·����ϳɸ�Ч���Ͷ�ũҩɱ�����( )��
��1��F����ʽΪ_____________________���ϳ�G�ķ�Ӧ������______________��
��2���й�A������˵����ȷ����__________(�����)��
a��A�DZ������ͬϵ��
b��A�ĺ˴Ź���������5����
c��ȼ�յ����ʵ���A�ͻ��������������������
d��A������ԭ�ӿ��ܴ���ͬһƽ����
e��A����ʹ����KMnO4��Һ��ɫ
��3��д����ӦB��C�Ļ�ѧ����ʽ��ע����Ӧ��������_________________________��
��4��д��C����������������ˮ��Һ�����·�����ַ�Ӧ�Ļ�ѧ����ʽ��__________________
��5��D����������·���ˮ������J��D J
д����������Ҫ���J������ͬ���칹��Ľṹ��ʽ(���Բ�����)��
________��________��________��_________��__________��
�ٱ�����������λ�ڼ�λ��ȡ������
����ˮ��������л��
��6����-������X��I��Ϊͬ���칹�壬��X����()��һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡѧ����ѧ�����߿�ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
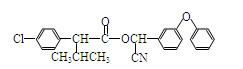
�������·����ϳɸ�Ч���Ͷ�ũҩɱ�����( ) ��
��1��F����ʽΪ_____________________���ϳ�G�ķ�Ӧ������______________��
��2���й�A������˵����ȷ����__________(�����)��
a��A�DZ������ͬϵ��
b��A�ĺ˴Ź���������5����
c��ȼ�յ����ʵ���A�ͻ��������������������
d��A������ԭ�ӿ��ܴ���ͬһƽ����
e��A����ʹ����KMnO4��Һ��ɫ
��3��д����ӦB��C�Ļ�ѧ����ʽ��ע����Ӧ��������_________________________��
��4��д��C����������������ˮ��Һ�����·�����ַ�Ӧ�Ļ�ѧ����ʽ��__________________
��5��D����������·���ˮ������J��D  J
J
д����������Ҫ���J������ͬ���칹��Ľṹ��ʽ(���Բ�����)��
________��________��________��_________��__________��
�ٱ�����������λ�ڼ�λ��ȡ������
����ˮ��������л��
��6����-������X��I��Ϊͬ���칹�壬��X����(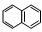 )��һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ�� ��
)��һȡ�������̼̼������д����Xͨ���ļ����Ӷ��ɵĸ߾���Y�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ����У��������һ���������ۻ�ѧ�Ծ��������棩 ���ͣ������
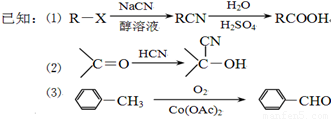
�������·����ϳɸ�Ч���Ͷ�ũҩɱ�����( ) ��
) ��

��1������ϵͳ������������B��������_____________���ϳ�G�ķ�Ӧ������__________��
��2���й�A������˵����ȷ����__________(�����)��
a��A�뱽��Ϊͬϵ��
b��A�ĺ˴Ź���������5����
c�����ȼ�յ����ʵ���A�ͻ��������������������
d��A������ԭ�ӿ��ܴ���ͬһƽ����
e��A����ʹ����KMnO4��Һ��ɫ
��3��д����Ӧ F+I��ɱ������Ļ�ѧ����ʽ��_____ ______________��
��4��д��C�ڸ��¸�ѹ��������������������������ˮ��Һ��ַ�Ӧ�Ļ�ѧ����ʽ��
____ _____ _____
��5��D����������·���ˮ������J��D 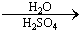 J
J
д����������Ҫ���J������ͬ���칹��Ľṹ��ʽ�� _��
�ٱ�����������λ�ڼ�λ��ȡ����������ˮ��������л���ۿ���������Ӧ��
��6��X��F��Ϊͬ���칹�壬X������������ȡ�����Һ���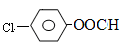 �ṹ������ϴ�������F����
�֡�
�ṹ������ϴ�������F����
�֡�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com