
���и�����������ָ�������д���������ǣ� ��
A����c(HCO3��)=0.1 mol·L-1����Һ�У�NH4+��AlO2����Cl����NO3��
B������ˮ�������c(H+)=1��10-12 mol·L-1����Һ�У�Fe2+��ClO����Na+��SO42��
C���ڼ������۲���H2����Һ�У�SO42����NO3����Na+��NH4+
D����ʹ��ɫʯ����ֽ��������Һ�У�SO32����CO32����Na+��K+

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��������������ĸ������档����˵������ȷ����
A����˾ƥ�־��н�����ʹ����
B��������Si3N4��Al2O3�������½ṹ�մ���Ʒ
C�����뺣�ڵĸ���բ����װһ��������ͭ��ɷ�ֹբ�ű���ʴ
D����ֹʹ�����һ�Ǧ�����Ϳ���������ɼ�������β����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������о������⣬���ܵõ�2-���������
A��2-��-1-��ϩ B��2-��-2-��ϩ
C��3-��-1-��Ȳ D��3��3-����-1-��Ȳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ�⾫��ͭ�������в���ȷ���ǣ� ��
A.��ͭ�壺����
B.���ʱ����������������Ӧ�������������ķ�ӦΪCu2++2e-====Cu
C.��ͭ������Na��Fe��Zn�����ʣ������Ե�����ʽ�����۵ף��γ�������
D.���ͭ�Ĵ��ȿɴ�99.95 %��99.98 %
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��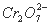 �Ĺ�ҵ���Է�ˮ����ɸ���Ⱦ���ŷ�ǰҪ�������´�������������ҵ��ˮ�м���������NaCl������ȣ�������Fe�����缫���е�⣬�Ӷ�ʹ��Һ��pH�������ߣ���ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr��OH��3��Fe��OH��3����������(��)���˻��ճ�������ˮ�ﵽ�ŷű���
�Ĺ�ҵ���Է�ˮ����ɸ���Ⱦ���ŷ�ǰҪ�������´�������������ҵ��ˮ�м���������NaCl������ȣ�������Fe�����缫���е�⣬�Ӷ�ʹ��Һ��pH�������ߣ���ˮ������ת��Ϊ���ԣ�����һ��ʱ����Cr��OH��3��Fe��OH��3����������(��)���˻��ճ�������ˮ�ﵽ�ŷű���
��1���ڵ������У���ҺpH�������ߵ�ԭ������ǣ� ��
�ٵ��ʱ��ˮ��������ϼ�С �ڵ��ʱH+����������ԭ ����Ϊ������Fe�����ܽ� ��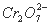 ת��ΪCr3+ʱ������H+ ��NaCl�ڵ��ʱת������NaOH
ת��ΪCr3+ʱ������H+ ��NaCl�ڵ��ʱת������NaOH
A.�� B.�ڢ� C.�ڢۢ� D.�٢ڢۢ�
��2������������Ӧ�ĵ缫��ӦʽΪ

��3��д��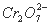 ���Cr3+�����ӷ���ʽ__________________________________��
���Cr3+�����ӷ���ʽ__________________________________��
��4��______________����ܡ����ܡ�������ʯī�缫���е�⡣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ�鷽���ɴӺ��������������ȡ���п��������Ե���Ȼ���

����˵��������ǣ� ��
A������(1)��Ҫ����װ�� B������(2)��Ҫ�õ���Һ©��
C������(3)��Ҫ�õ����� D������(4)��Ҫ����װ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��51.2gCu��ȫ��������Ũ�����У��ռ��������������NO��N2O4��NO2���Ļ���ﹲ0.8mol����Щ����ǡ���ܱ�500ml 2mol/LNaOH��Һ��ȫ���գ����ɺ�NaNO3��NaNO2������Һ������NaNO2�����ʵ���Ϊ�� ��
A��0.2mol B��0.6mol C��0.8mol D��1.0mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڹ�ҵ������˵���У�����ȷ����(����)
A����ҵ�ϣ��ý�̿�ڵ�¯�л�ԭ��������õ������ʵĴֹ�
B��������ͨˮ�����Ҫԭ����ʯ��ʯ��ʯӢ�ʹ���
C����ҵ�Ͻ���ͭ���о�����Ӧ����ͭ�����ڵ�Դ������
D���ڸ�¯�����ķ�Ӧ�У�һ����̼����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)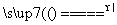 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
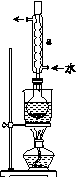
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O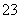 ��I2===S4O
��I2===S4O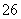 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
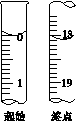
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO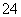 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com