


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��19���ͳ���Ӣ����ѧ�ҵ��������ԭ��ѧ˵ |
| B��1911�꣬¬ɪ�����ݦ�����ɢ����������˴��˵�ԭ�ӽṹģ�� |
| C����ķ������ԭ���д��ڵ��ӣ������ԭ�ӽṹ���ģ�� |
| D��20���ͳ�����ѧ��ָ��������������ѧ��������������ӵ��˶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���ۢ� | D���٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 B��__
B��__ _______��C��_________��D��_________��E��_________��
_______��C��_________��D��_________��E��_________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


 C�е���������Ҫ��_
C�е���������Ҫ��_ _________����������ӵ�ʵ�����Ϊ______________
_________����������ӵ�ʵ�����Ϊ______________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
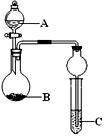
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| W | X |
| Y | Z |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HF��HCl��HBr��HI�����ȶ��Ժͻ�ԭ�Դ��������μ��� |
| B��O2�D�뾶��F�D��С |
| C��Na��Cs���ڵڢ�A��Ԫ�أ�Csʧ����������Na��ǿ |
| D���������ڷǽ���Ԫ�غ���������Դ�����������ǿ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com