
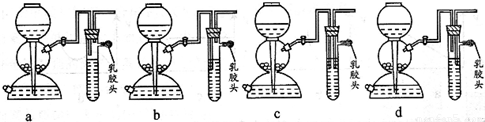
��1����ȼ�������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á���ͼ��װ���������õ���___________��
��2��ȡһ�����ı�����ˮ��CaCO3��ĩ��ϣ��۲쵽�������ݣ���ˮ�Ļ���ɫ��ȥ����ֹ��ȡ�ϲ������Һ�ķݣ��ֱ��������ʵ�飺
�� һ�ݵμ����ᣬ���������� ��
��
�� һ�ݵμ�NaOH��Һ�����ְ�ɫ����
�� һ���þƾ��Ƽ��ȳ��ְ�ɫ����
�� ����ɫ����������ķ���Һ������������ɫ
������ʵ�������Ʋ�ó�����Һ����Ҫ������ ��
ʵ��ٿ����� ����ʽ��ʾ��
������ˮ��CaCO3��ĩ������Ӧ�Ļ�ѧ����ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�þ�Ļ�������й㷺��;����ش��й�þ���������⣺
(1) ����þ�ڿ�����ȼ�յ���Ҫ�����ǰ�ɫ��_________��������������_____���ѧʽ����
(2) ���ʵ���Ϊ0.10 mol��þ����ֻ����CO2��O2��������������ȼ��(���ﲻ��̼��
þ������Ӧ�������ڹ������ʵ�����������Ϊ��
A��3.2g B��4.0g C��4.2g D��4.6g
(3)������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㡣Mg2Cu��һ��
����Ͻ�350��ʱ��Mg2Cu��H2��Ӧ������MgCu2�ͽ���һ�ֽ���Ԫ�ص��⻯��(����
�����������Ϊ0.077��Mg2Cu��H2��Ӧ�Ļ�ѧ����ʽΪ ��
��4����ȼ�������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�á�
��ͼ��װ���������õ���___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ���ݸ���ѧ��һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com