
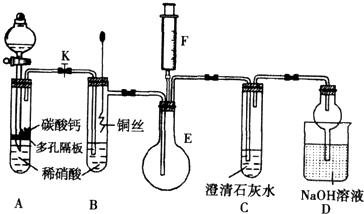
��11�֣� ijУ����С��Ϊ��̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����������ʵ�飬װ����ͼ��ʾ(����װ�ú̶�װ�þ�����ȥ)��ͼ��KΪֹˮ��(���ڹر�״̬)��F��һ��յ�ע������

��ش��й����⣺
(1) ���װ��A��Ŀ���� ��
Ϊ�ﵽ��Ŀ�ģ�Ӧ���еIJ����Ǵ�K���Ҵ�Һ©����������װ��C�в���
ʱ���ر�K��
(2) �����(1)�еġ���������װ��B��ͭ˿����ϡ���ᣬ����֮���۲쵽װ��B�е�
������ ��
B�з�Ӧ�����ӷ���ʽΪ�� ��
(3) װ��E��F�������� ��Ϊʵ�ִ����ã������������ ��
(4) װ��D�����������ն���ĵ��������ֹ��Ⱦ���������� �Ĺ��ܡ�
(1)�������ɵĶ�����̼������װ���ڵĿ����Ͼ�������NO��������Ӧ���ɶ����������������Ĺ۲�������š� ��2�֣� ��ɫ������1�֣�
(2)ͭ˿����������ݣ�ϡ����Һ������Ϊ��ɫ����Һ��Ϊ��ɫ��2�֣�
3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��2�֣�
(3)��֤��ɫ����ΪNO����1�֣� ��ע����F�еĿ�������E�л�E�е���ɫ�������뵽ע�����С���2�֣� (4)��ֹ��Һ��������1�֣�
����������1������װ���к��п������ܰ�NO��������NO2���Ӷ�����ʵ�顣������Ҫ�������ɵĶ�����̼������װ���ڵĿ����Ͼ�������NO��������Ӧ���ɶ����������������Ĺ۲�������š������ʯ��ˮ������CO2��������ɫ�������ݴ˿����жϡ�
��2��ϡ������������ԣ��ܰ�ͭ��������������ͭ��NO��ˮ�������������ͭ˿����������ݣ�ϡ����Һ������Ϊ��ɫ����Һ��Ϊ��ɫ���йصķ���ʽΪ3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��4������NO���ױ���������NO2������EF����������֤��ɫ����ΪNO�ġ�����IJ����ǽ�ע����F�еĿ�������E�л�E�е���ɫ�������뵽ע�����С�
��5��NO2��������ˮ�����Ի��з�ֹ��Һ���������á�


 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 1 |
| 2 |
| 1 |
| 2 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
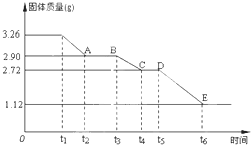 ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4?yH2O����������ʵ�飺��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ������˵��������ǣ�������
ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4?yH2O����������ʵ�飺��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ������˵��������ǣ�������| A��t5��t6ʱ��ι������������ԭ���Dz�����SO2��O2�������� | B��t6��õ��Ĺ�����CaSO4 | C��t2��t3ʱ��ι���Ļ�ѧʽΪ2CaSO4?H2O | D��x��y=2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������һ����;�dz��㷺�IJ�Ʒ��
��1��25��ʱ��Ksp(CaSO4)=7.10��10-5����1L0.1mol��L��1CaCl2��Һ�м���1L0.2mol��L��1��Na2SO4��Һ����ַ�Ӧ�����Ϻ���Һ������仯���Բ��ƣ���Һ��Ca2+���ʵ�����Ũ��Ϊ �� mol��L��1��
��2��ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ������(xCaSO4��yH2O)��������ʵ�飺��������������м��ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ��

x ��y =____��_____��
�� t2��t3ʱ��ι���Ļ�ѧʽΪ �� ��
�� t5��t6ʱ��ι������������ԭ���Dz������������壬����һ����ʹƷ����Һ��ɫ�����ʱ�����������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�����и�����ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��10�֣�(1)�����£�ijˮ��ҺM�д��ڵ������У�Na+��A2-��HA-��H+��OH-�����ڵķ�����H2O��H2A����������ش��������⣺
��д����H2A�ĵ��뷽��ʽ__________________________��
������ҺM��2 mol��L-1NaHA��Һ��2mol��L-1NaOH��Һ�������϶��ã�����ҺM��pH ____7 ���>������<����=��������ҺM�и�����Ũ�ȹ�ϵ��ȷ���� ��
A.c(Na��)��c(A2��)��c(OH��) ��c(H��)
B. c(HA��) ��c(H2A) ��c(H��)��c(OH��)
C.c(A2��)��c(HA��) ��c(H2A)��1 mol��L-1
D. c(A2��)��c(HA��)��c(OH��)��c(Na��)��c(H��)
(2)����ʱ���������Ƶ��ܶȻ�KSP =4.7��10-6, ����ʱ��9 mL0.02 mol��L��1���Ȼ�����Һ��1 mL pH=13������������Һ��Ϻ�(��Һ�����ֱ�ӼӺ�),��Һ��___ ��������(��С����ޡ�)��
(3) ijУ����С��Ϊ�ⶨ�Ѳ�����ˮ����ʯ�����ɣ�xCaSO4��yH2O����������ʵ��:��������ȣ�������ʣ�����������ʱ��仯��ͼ��ʾ��
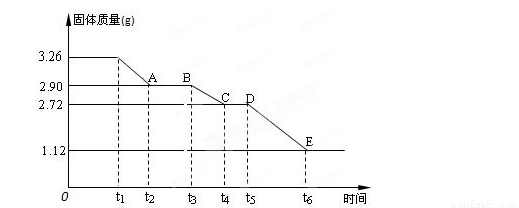
��x:y= ��t2~t3ʱ��ι���Ļ�ѧʽΪ ��t5~t6ʱ��ι������������ԭ���Dz������������壬����һ����ʹƷ����Һ��ɫ�����ʱ����������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com