
I�����ǻ��������һ��ǿЧ�ĵ�����ϣ���Һ����ʾ����ҵ�н������о��㷺���ṹ��ʽ����ͼ��
��1�����л���ķ���ʽΪ �������������ŵ������� ��
��2�����л����ܷ����ķ�Ӧ�����ǣ���д���ţ� ��
A��������Ӧ B����ȥ��Ӧ C���Ӿ۷�Ӧ D��ˮ�ⷴӦ
II�������廯����C10H10O2�����µ�ת����ϵ��
��֪E��ʹBr2/CCl4��Һ��ɫ��
��3����ֱ�д��A��C�Ľṹ��ʽ��A �� C ��
��4�����л���F��C��Ϊͬ���칹�������л���B��Ϊͬϵ������������F�� �֣�������˳���칹����
��5����д��D��E��Ӧ�Ļ�ѧ����ʽ�� ��
��15�֣�
��1��C9H8O3��2�֣�ԭ��˳�����0�֣�
���ӣ��ǻ� �Ȼ� ����2�֣���1�֣��д���0�֣�
��2��A C ��2�֣�ȫ��2�֣�ѡ��1����1�֣��д���0�֡���
��3�� CH2=CHCOOCH3����4�֣���2�֣�
CH2=CHCOOCH3����4�֣���2�֣�
��4�� 3 ��2�֣�
��5�� ��3�֣�©������1�֣�
��3�֣�©������1�֣�
���������������1���۲���֪�ṹ��ʽ����������HO������C6H4������CH=CHCOOH�����ֹ��ɣ���������ʽΪC9H8O3��������Ϊ�ǻ���̼̼˫�����Ȼ�������Ԫ�صĹ�����ֻ���ǻ����Ȼ�����2������ɿ������л�����ȼ�գ��ӹ����ſ������л���ɱ������е����������ɷۺ�ɫ��Ҳ��ʹ���Ը��������Һ��ɫ������ܷ���������Ӧ����A��ȷ�����ڱ����ṹ�ȶ������ǻ����ܷ�����ȥ��Ӧ����B�������ں���̼̼˫�����������һ�������·����Ӿ۷�Ӧ����C��ȷ������������������л��ﲻ�ܷ���ˮ�ⷴӦ����D����3����ת����ϵͼ�ƶϣ�A��B�ֱ������������ᣬ���������غ㶨���ƶ�A��ˮ�����B�ķ���ʽΪC3H4O2����B�Ľṹ��ʽΪCH2=CHCOOH��1���ӱ�ϩ���1���ӶԼ����ӷ���������Ӧ����������1����H2O��1����A���ɴ����ƿɵ�A�Ľṹ��ʽ����ϩ����״���Ũ�������ʱ����������Ӧ����CΪ��ϩ��������ṹ��ʽΪCH2=CHCOOCH3����4�������⣬F���ڲ��������ᣬC�ķ���ʽΪC4H6O2���ɴ��ƶ�Fֻ��1���Ȼ���ʣ�µ�ԭ����Ϊ��C3H5����B��ͬϵ���ƶϡ�C3H5���ܵĽṹ��CH2=CHCH2����CH2=C(CH3)������CH=CHCH3����˷���������F�����֣��ֱ���CH2=CHCH2COOH��CH2=C(CH3)COOH��HOOCCH=CHCH3����5���Լ����Ӻ��б�������һ��������������H2�����ӳɷ�Ӧ�����ɵ�DΪ4������������4������������Ũ���Ṳ��ʱ������ȥ��Ӧ����ȥ�ǻ���2��6��̼ԭ���ϵ���ԭ�ӣ�����H2O��4��������ϩ��
���㣺�����л���ϳ����ƶϣ��漰�л���ķ���ʽ�������������ʡ��ṹ��ʽ��ͬ���칹�塢��ѧ����ʽ�ȡ�


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
ú�����ɵõ���̿��ú���͡��ְ�ˮ�ͽ�¯������̿��ͨ������;����ȡ������ϩ�Ȼ�����Ʒ�����¿�ͼ��ʾ��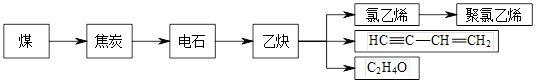
44.д���ɵ�ʯ��ȡ��Ȳ�Ļ�ѧ����ʽ________________________________��
45.������ϩ��Ʒ����ɰ�ɫ��Ⱦ��������ϩ�Ľṹ��ʽΪ_________________��
C2H4O���Ƿ���ȩ������________________�����Լ����ƣ������顣
46.��ú�����п��Է����һ����Ҫ��Һ̬����������д������Һ���������ڵ�����·�����Ӧ�Ļ�ѧ����ʽ__________________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��13�֣���֪����A�����ԣ���������¿�ͼ�ش�����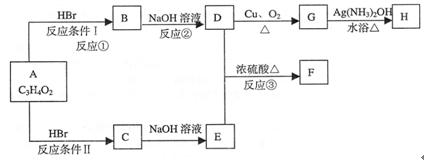
FΪ��ԭ����ɵĻ�״�ṹ
��1��A�Ľṹ��ʽΪ ��2�֣�
��2���٢ڢ۵ķ�Ӧ���ͷֱ�Ϊ �� �� ��3�֣�
��3��������B�к��й����ŵ������� ��2�֣�
��4��C����E�Ļ�ѧ����ʽ ��2�֣�
��5��G����H�Ļ�ѧ����ʽ ��2�֣�
��6��д��C��ͬ���칹���������������ʵĽṹ��ʽ ��
����д2�֣���2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
������ѧ��R.F.Heck��������Heck��Ӧ�����2010��ŵ������ѧ�����÷�Ӧԭ�����£� ��XΪ±ԭ�ӣ�RΪȡ������
��XΪ±ԭ�ӣ�RΪȡ������
����Heck��Ӧ�ϳ�E��·�����£�
��1����֪A Ϊ���ᣬ A��B�ķ�Ӧ������ ��0.1mol��A�������Ľ���Na��Ӧ����H2 L����״���£���
��2��д����ӦI�ķ���ʽ ��ע��������
��3������A����ʽ�����������������֣����е�һ�־���������Ӧת����B����֪B���ӵĺ˴Ź������������ַ壬B�Ľṹ��ʽΪ ��
��4����Ӧ��ΪHeck��Ӧ����E�Ľṹ��ʽΪ ��
��5��A��һ��ͬ���칹��F���������з�ӦҲ���Ƶ�C��
ʵ���Ҽ��鷴Ӧ���Ƿ���ȫ���õ��Լ��ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л���A��G��ת����ϵ����ͼ��ʾ������A��һԪ��״�������˴Ź���������ֻ��1���壻F�ĺ˴Ź�����������3���壬�������Ϊ2��2��3��G��һ�ֺϳ�����֬��һ����Ҫԭ�ϡ�
��֪��(RΪ����) ��
��RCOOH RCH2OH
RCH2OH
��ش��������⣺
��1��C�����������ŵ������� ����Ӧ�ܵķ�Ӧ������___________________��
��2����E��һ�������·������۷�Ӧ���ɸ߷��ӻ����д���������ֵĽṹ��ʽ��
_____________________________��_____________________________��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ��
��4����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________��
��5���л���Y��E��Ϊͬ���칹�壬���Ҿ�����ͬ�Ĺ������������Ŀ��д�����з���������Y�Ľṹ��ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��6�֣�ijǿ������ҺX�к���Ba2����Al3����SiO32����NH4����Fe2����Fe3����CO32����SO42����NO3���е�һ�ֻ������ӣ�ȡ����Һ��������ʵ�飬��ʵ������ת����
����������Ϣ����ش��������⣺
��1����ҺX�г���H����Al3����NH4����SO42����϶������е������� ������ȷ���Ƿ��е������� ����Ҫȷ������ȷ�����������Ƿ���ڣ���ɿ������ǣ� ��
��2������E�Ļ�ѧʽΪ ��
��3����Ӧ�١��ڡ��ۡ����У�������������ԭ��Ӧ���� ������ţ�
��4��д�����������������A�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
(12��)��50mL0��50mol/L������50mL0��55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2���ձ���������ֽ���������� ��
��3�������60mL0��50mol/L������50mL0��55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)���������� ��
��4������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ�� ��ƫС��������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�������
�� ��������Ϊ0.365�ܶ�Ϊ1.19g/cm3��Ũ���ᣬ�������������ʵ���Ũ�ȡ�
�� ȡ��������50mlϡ�ͳ�100 mL��Һ������15g CaCO3��ֲ�������������ڱ�״�����Ƕ��٣�
�� ����������������ȫ��ͨ��500mLŨ��Ϊ0.4mol/L��NaOH��Һ����ȫ��Ӧ��������Һ�������ʲô���������ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶���һѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���л�ѧ��Ӧ��H������
A��NaOH(aq)��HCl(aq)��NaCl(aq )��H2O(l)����H1
B�� NaOH(aq)��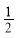 H2SO4(aq)��
H2SO4(aq)��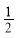 Na2SO4(aq)��H2O(l)����H2
Na2SO4(aq)��H2O(l)����H2
C��CH3COOH(aq)��NaOH(aq)��CH3COONa (aq )��H2O(l)����H3
D��NaOH(aq)��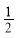 H2SO4(Ũ)��
H2SO4(Ũ)��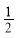 Na2SO4(aq)��H2O(l)����H4
Na2SO4(aq)��H2O(l)����H4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com