
(1)���ݽṹ���л�����з��࣬�����ڶ������ʵ����ա�
�������л������ڷ���������____________(����ĸ)�����뱽�Ĺ�ϵ��_____________��д�������巢����Ӧ�Ļ�ѧ����ʽ________________________________��Ԥ��÷�����____________(��ܡ����ܡ�)�������෴Ӧ��
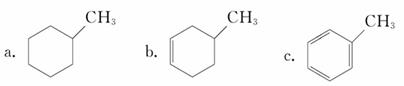
�������л����������������___________(����ĸ)��
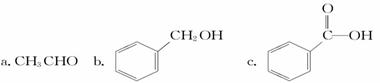
�������л��������������___________(����ĸ)��
�ᣮ��֬ �⣮��ά�� c��������
(2)������X�Ľṹ��ʽΪ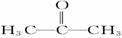
��X������___________����ԭ�ӡ�
��X��һ��ͬ���칹��Z�Ľṹ��ʽΪH2C��CH��CH2OH����д��Z��Br2�����ӳɷ�Ӧ�Ļ�ѧ����ʽ________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ�Ӧ����ʽ��д��ȷ���ǣ� ��
A��ϡ�����Ba(OH)2��Һ��Ӧ Ba2++OH-+ H++ SO42-==BaSO4��+ H2O
B��������SO2ͨ��NaOH��Һ�� SO2 + 2OH- ==SO32-+ H2O
C������Fe3+�� Fe3+ + 3SCN-- == Fe(SCN)3��
D��SO2ʹ��ˮ��ɫ SO2 + Br2 +2H2O == SO42-+2Br-+ 4H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�п�Ժ��CO2�ϳ��˿ɽ������Ͼ۶�����̼���������˵���������� (����)��
A���۶�����̼������ͨ���ۺϷ�Ӧ�Ƶõ�
B���۶�����̼������ɱ���Ϊͬ��������
C���۶�����̼������ɱ������ڴ�����
D���۶�����̼���ϵ�ʹ�û������ɫ��Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ڹ�������������Ӧ��ֻ����һ��һ�ȴ�������( )
A��CH3CH2CH2CH3 B��CH3CH(CH3)2 C��CH3C(CH3)3 D��(CH3)2CHCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɫ��ѧ�����У�����״̬�Ƿ�Ӧ����ԭ��ȫ��ת��Ϊ���ƵõIJ����ԭ��������Ϊ100%������CH3C��CH�ϳ�CH2===C(CH3)COOCH3�Ĺ����У���ʹԭ�������ʴﵽ��ߣ�����Ҫ�����ķ�Ӧ����(���� )
A��CO2��H2O B��CO��CH3OH
C��CH3OH��H2 D��H2��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵��X�ĵ縺�Ա�Y�Ĵ���� ( )
A.��H2����ʱX���ʱ�Y��������
B.X������������Ӧˮ��������Ա�Y������������Ӧˮ���������ǿ
C.Xԭ�ӵ�������������Yԭ��������������
D.X���ʿ���Y�����⻯�����û�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��X��Y��Z������X��Yλ��ͬһ���壬Y��Zλ��ͬһ���ڡ�Xԭ�ӵ�����������������Ӳ�����3����Zԭ�ӵĺ����������Yԭ����1�����бȽ���ȷ���ǣ� ��
A. ��̬�⻯����ȶ��ԣ�Z < Y < X B.���������ˮ�������ԣ�Z > Y
C. ԭ�Ӱ뾶��Z < Y < X D. Ԫ�طǽ����ԣ�Z> Y > X
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йػ�ѧ������ȷ���� �� ��
����ϩ�����ʽC2H4 ���Ҵ��Ľṹ��ʽC2H6O
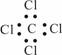 |
�����Ȼ�̼�ĵ���ʽ ����Ȳ�Ľṹ��ʽCHCH
������ĽṹʽCH3CH3 ����ȩ�Ľṹ��ʽCH3COH
A��ȫ�� B��ȫ�� C���ۢܢ� D���ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͼ1��ͼ2�ֱ���1s���ӵĸ��ʷֲ�ͼ��ԭ�ӹ��ͼ�������й���ʶ��ȷ���� (����)��
ͼ1��ͼ2�ֱ���1s���ӵĸ��ʷֲ�ͼ��ԭ�ӹ��ͼ�������й���ʶ��ȷ���� (����)��
A��ͼ1�е�ÿ��С�ڵ��ʾ1������
B��ͼ2��ʾ1s����ֻ���������ڳ���
C��ͼ2����1s�����Բ�Σ��������Գ���
D��ͼ1�е�С�ڵ��ʾijһʱ�̣������ں���������λ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com