
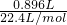 =0.04mol������ʮ����˷�ȷ��һ�������Ͷ������������ʵ���֮�ȣ�
=0.04mol������ʮ����˷�ȷ��һ�������Ͷ������������ʵ���֮�ȣ�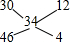 ������һ�������Ͷ������������ʵ���֮��=12��4=3��1����һ�����������ʵ�����0.03mol���������������ʵ�����0.01mol������ת�Ƶ����غ��ͭ�����ʵ���=
������һ�������Ͷ������������ʵ���֮��=12��4=3��1����һ�����������ʵ�����0.03mol���������������ʵ�����0.01mol������ת�Ƶ����غ��ͭ�����ʵ���=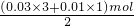 =0.05mol��
=0.05mol��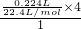 =0.04mol������ԭ���غ�֪���������ʵ�����0.04mol��
=0.04mol������ԭ���غ�֪���������ʵ�����0.04mol��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�Cu��Fe�Ͻ����ڳ�ʱ�����ڿ����У����������һ������Ĥ���ɷ�ΪFe2O3��CuO�����ֽ�������ʵ�飨��������������ڱ���²�ã���
�ٽ��˺Ͻ��5.76g�����ձ��У�Ȼ��ϡH2SO4�����������������ռ�����������Ϊ672mL�����˵�dz��ɫ��ҺA����������B��
�ڽ�����BͶ�뵽һ��Ũ�ȵ�HNO3�У���ȫ�ܽ⣬��NO��NO2�Ļ������896mL�����ⶨ��ͬ��ͬѹ�´˻�����������������ܶ�Ϊ17��
�۽�����������Һ���뵽ͬŨ��������HNO3�У�����ˮ���ռ�һ��ƿ���壬������ƿ��ͨ��224mL O2������ǡ����ȫ����ˮ��
��1��A�д��ڵ��������� ��
��2��896mL���������NO��NO2�����ʵ���֮��Ϊ ��
��3��B�ĵ���Ϊ ������Ϊ g��
��4�����б�HNO3�����˵������ӵ����ʵ���Ϊ mol��
��5�� �˺Ͻ������Ԫ�ص�����Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������б����������һ�и�����ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ�������
��8�֣�Cu��Fe�Ͻ����ڳ�ʱ�����ڿ����У����������һ������Ĥ���ɷ�ΪFe2O3��CuO�����ֽ�������ʵ�飨��������������ڱ���²�ã���
�ٽ��˺Ͻ��5.76g�����ձ��У�Ȼ��ϡH2SO4�����������������ռ�����������Ϊ672mL�����˵�dz��ɫ��ҺA����������B��
�ڽ�����BͶ�뵽һ��Ũ�ȵ�HNO3�У���ȫ�ܽ⣬��NO��NO2�Ļ������896mL�����ⶨ��ͬ��ͬѹ�´˻�����������������ܶ�Ϊ17��
�۽�����������Һ���뵽ͬŨ��������HNO3�У�����ˮ���ռ�һ��ƿ���壬������ƿ��ͨ��224mL O2������ǡ����ȫ����ˮ��
��1��A�д��ڵ��������� ��
��2��896mL���������NO��NO2�����ʵ���֮��Ϊ ��
��3��B�ĵ���Ϊ ������Ϊ g��
��4�����б�HNO3�����˵������ӵ����ʵ���Ϊ mol��
��5���˺Ͻ������Ԫ�ص�����Ϊ g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com