
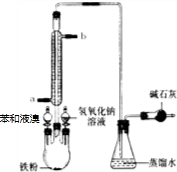
��9�֣���ͼװ�â���ʵ���������������ij���װ�ã�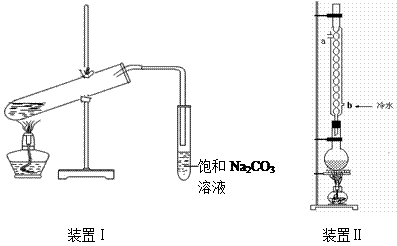
��װ��1���Թܿ��Ƿ�Ҫ��������
ʵ�������õ�ijЩ�Լ������������������£�
| �� �� |
| �е�/�� | �ܶ�/g��cm��3 | ||
| �� �� | ��114 | 78 | 0.789 | ||
| �� �� | 16.6 | 117.9 | 1.05 | ||
| �������� | ��83.6 | 77.5 | 0.900 | ||
| 98%H2SO4 | 10 | 338 | 1.84 |
 ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ���������
��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ��������� �������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
�������ã� ��������Ȼ�����_____�ڣ���ϡ����¡��������� �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| H | + 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1������ƿ�з�����Ӧ�Ļ�ѧ����ʽ
��1������ƿ�з�����Ӧ�Ļ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ��12���¿���ѧ�Ծ� ���ͣ�ʵ����
��9�֣���ͼװ�â���ʵ���������������ij���װ�ã�

��װ��1���Թܿ��Ƿ�Ҫ��������
ʵ�������õ�ijЩ�Լ������������������£�
|
�� �� |
�۵�/�� |
�е�/�� |
�ܶ�/g��cm��3 |
|
�� �� |
��114 |
78 |
0.789 |
|
�� �� |
16.6 |
117.9 |
1.05 |
|
�������� |
��83.6 |
77.5 |
0.900 |
|
98%H2SO4 |
10 |
338 |
1.84 |
�ش��������⣺
��1�������CH3CO18OH��CH3CH2OH��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ���ڷ�Ӧ����������б��18O��λ�ã� ��ŨH2SO4�������� ��
��2��Ҫ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ������������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
��3������װ�â��������������IJ��ʣ���ϱ����е����ݣ�˵����װ�ÿ���������������ʵ�ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�߶���ѧ����ĩ��ѧ�Ծ� ���ͣ�ʵ����
��9�֣���ͼװ�â���ʵ���������������ij���װ�ã�
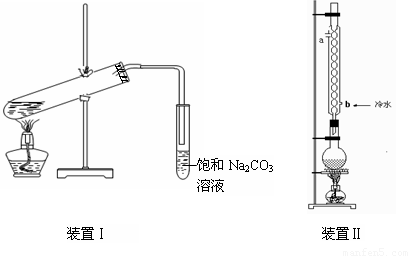
ʵ�������õ�ijЩ�Լ������������������£�
|
�� �� |
�۵�/�� |
�е�/�� |
�ܶ�/g��cm��3 |
|
�� �� |
��114 |
78 |
0.789 |
|
�� �� |
16.6 |
117.9 |
1.05 |
|
�������� |
��83.6 |
77.5 |
0.900 |
|
98%H2SO4 |
10 |
338 |
1.84 |
�ش��������⣺
��1�������CH3CO18OH��CH3CH2OH��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��ŨH2SO4�������� ��
��2��Ҫ��װ�â����Ҳ�С�Թ��з��������������Ӧ���еIJ����ǣ�����С�Թܣ������Һ���� �����������ƣ������������ã� ��������Ȼ�����_____�ڣ���ϡ����¡���������
��3������װ�â��������������IJ��ʣ���ϱ����е����ݣ�˵����װ�ÿ���������������ʵ�ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ϻ��к���������ڶ���ģ�⿼�Ի�ѧ���� ���ͣ�ʵ����
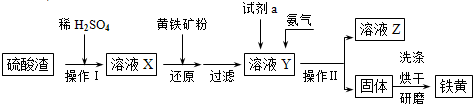
ʵ��������ȡ����NaHCO3��NH4Cl��ʵ�鲽�����£�
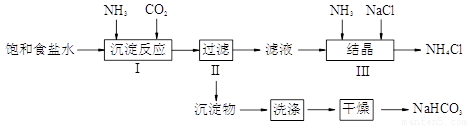
��ش�
��1������I��III�����õ�����������װ�ÿ�����ʵ�����ư�������_________��������ţ�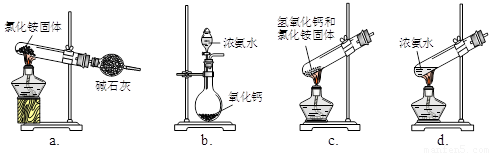
��2��д������I�з����Ļ�ѧ��Ӧ����ʽ
_______________________________________________________��
������ͼװ�ý��иó�����Ӧ��ʵ��ʱ�����ȴ�_____���a������b����c������ͨ��_____���塣

��3������III����Һ��ͨ�백��������ϸСʳ�ο�������ʹNH4Cl���嵥���ᾧ������
�ٴ˴�ͨ�백����������_________��
a������NH4+��Ũ�ȣ�ʹNH4Cl���������
b��ʹNaHCO3���������
c��ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
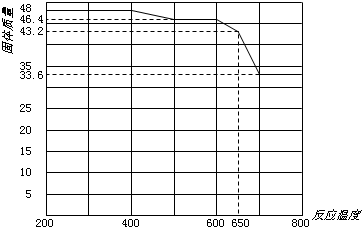
����֪����������ʲ�ͬ�¶��µ��ܽ��������ͼ��ʾ���ᾧʱ�˲���___________��������ᾧ�����½ᾧ������
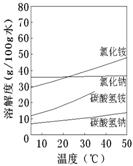
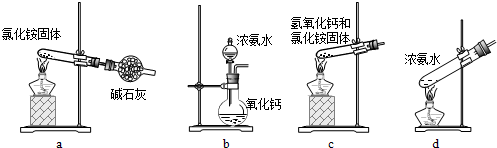
���������������ͼ��ʾ��װ������֤���õľ����к���NH4+��

���������ȡ����������Ӳ���Թܣ��Ծ��岿λ���ȡ�����_____
���A����B����������______________��ֽ���۲�����
��4���밴��ʾ���NaHCO3����������ʵ����ơ�
�Լ������ᡢ����ʯ��ˮ������������Һ������ˮ��
������������ƽ���ձ���©��������������������[��Դ:ZXXK]
�� _________________����ˮ������__________________________��
�ڹ��ˡ�ϴ�ӡ���ɣ���ȴ��������������_____________������д�������ƣ���
�ۼ��㡣�����ݴ���ʱ�����ù�������1.977 g����Ϊ1.971 g���ɴ˲�����������Ϊ_____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com