
| ʵ�鲽���ʵ������ | ʵ����� |
| �۲���Һ����ɫ | ��ԭҺ��һ�������������ǣ� Cu2+��MnO4-��Fe2+ |
| ��ȡ��������Һ��������������ᣬ���������ɣ����õ���ɫ��Һ | ��ԭҺ��һ�������������ǣ� Mg2+��Ag+��Ba2+��Al3+��I-��SiO32- һ�����������ǣ� CO32-��K+ |
| ���ڢ����õ���Һ���ټ��������̼�������Һ�����������ɣ�ͬʱ������ɫ����A | ��ԭҺ�л�һ�����е������ǣ� AlO2-���ɳ���A�����ӷ���ʽΪ�� AlO2-+HCO3-+H2O=CO32-+Al��OH��3�� |
| �����ڢ�������Һ������μ�������������Һ������������Ҳ���������ɣ�ͬʱ������ɫ����B | �ܰ�ɫ����B��һ�����У� BaCO3���ܺ��У� BaSO4 |
���� I���۲���Һ����ɫ������һ����������ɫ���ӣ�Cu2+��MnO4-��Fe2+��
��ȡ��������Һ��������������ᣬ���������ɣ�������Ϊ������̼������Һ��һ������CO32-��һ����������CO32-���ӷ�Ӧ��Mg2+��Ag+��Ba2+��Al3+���ӣ��õ���ɫ��Һ����һ��������I-��SiO32-���ٸ�����Һ�ʵ������ж�ԭ��Һ��һ������Ψһ������K+��
���ڢ�������Һ���ټ������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ����A��˵����Һ��һ�����������ӣ�ԭ��Һ��һ������AlO2-��
IV���ڢ�������Һ�к��й�����̼����泥��������Ba��OH��2��Һ�����������Ȼ��а������ɣ���ɫ��������Ϊ̼�ᱵ��̼�ᱵ�����ᱵ�Ļ���
�Դ˽����⣮
��� �⣺��1��I���۲���Һ����ɫ������һ����������ɫ���ӣ�Cu2+��MnO4-��Fe2+��
��ȡ��������Һ��������������ᣬ���������ɣ�������Ϊ������̼������Һ��һ������CO32-��һ����������CO32-���ӷ�Ӧ��Mg2+��Ag+��Ba2+��Al3+���ӣ��õ���ɫ��Һ����һ��������I-��SiO32-���ٸ�����Һ�ʵ������ж�ԭ��Һ��һ������Ψһ������K+��
���ڢ�������Һ���ټ������NH4HCO3��Һ�����������ɣ�ͬʱ������ɫ����A��˵����Һ��һ�����������ӣ�ԭ��Һ��һ������AlO2-����Ӧ�����ӷ���ʽΪ��
IV���ڢ�������Һ�к��й�����̼����泥��������Ba��OH��2��Һ�����������Ȼ��а������ɣ���ɫ��������Ϊ̼�ᱵ��̼�ᱵ�����ᱵ�Ļ���
�ʴ�Ϊ��
| ʵ�鲽���ʵ������ | ʵ����� |
| �۲���Һ����ɫ | ��Cu2+��MnO4-��Fe2+ |
| ��ȡ��������Һ��������������ᣬ���������ɣ����õ���ɫ��Һ | ��Mg2+��Ag+��Ba2+��Al3+��I-��SiO32-��CO32-��K+ |
| ���ڢ����õ���Һ���ټ��������̼�������Һ�����������ɣ�ͬʱ������ɫ����A | ��AlO2-��AlO2-+HCO3-+H2O=CO32-+Al��OH��3�� |
| �����ڢ�������Һ������μ�������������Һ������������Ҳ���������ɣ�ͬʱ������ɫ����B | ��BaCO3��BaSO4 |
���� ���⿼��������ƶϣ�Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬��Ŀ�漰���ӹ��桢�������ӵļ��飬��Ŀ�Ѷ��еȣ�����������ϴ�ע�����ճ������ӵ����ʼ����鷽������ȷ���ӷ�Ӧ���������������ӹ����������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ�ô��� | B�� | �ı�Ũ�� | C�� | �����¶� | D�� | �����¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
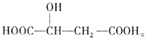 ����˵������ȷ���ǣ�������
����˵������ȷ���ǣ�������| A�� | ƻ������һ�������������Ҵ�������Ӧ | |
| B�� | ƻ������һ���������ܷ�����������Ӧ | |
| C�� | ƻ������һ���������������ᷢ����Ӧ | |
| D�� | 1molƻ������Na2CO3��Һ��Ӧ�������3mol Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ�鲽����ʵ������ | ʵ����� |
| �۲���Һ����ɫ�� | ��ԭ��Һ��һ��������������Cu2+��MnO4-��Fe2+ |
| ��ȡ��������Һ��������������ᣬ���������ɣ����õ���ɫ��Һ | ��ԭ��Һ��һ��������������Mg2+��Ag+��Ba2+��Al3+��I-��SiO32-��һ�����е�������CO32-��K+ |
| ���ڢ�������Һ���ټ��������̼�������Һ�����������ɣ�ͬʱ������ɫ����A | ��ԭ��Һ�л�һ�����е�������AlO2-�����ɳ���A�����ӷ���ʽΪAl3++3HCO3-=Al��OH��3��+3CO2�� |
| �����ڢ�������Һ������μ�������������Һ������������Ҳ���������ɣ�ͬʱ������ɫ����B | �ܰ�ɫ����B��һ������BaCO3�����ܺ���BaSO4 |
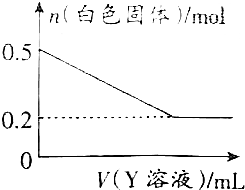
 ��3���û�ѧ��ȤС���ͬѧΪ�˽�һ��ȷ��B�ijɷ֣�ȡһ������ϴ�Ӻ��B��Y��Һ��Ӧ����ɫ��������ʵ�����Y��Һ���֮��Ĺ�ϵ��ͼ��ʾ��
��3���û�ѧ��ȤС���ͬѧΪ�˽�һ��ȷ��B�ijɷ֣�ȡһ������ϴ�Ӻ��B��Y��Һ��Ӧ����ɫ��������ʵ�����Y��Һ���֮��Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
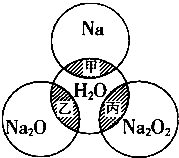 ��ͼ��ʾ����ԲȦ�ཻ����Ӱ���ֱ�ʾԲȦ�ڵ�����������ķ�Ӧ����֪�Ƽ�������������ʵ�����Ϊ0.1mol��ˮ������Ϊ100g������˵����ȷ���ǣ�������
��ͼ��ʾ����ԲȦ�ཻ����Ӱ���ֱ�ʾԲȦ�ڵ�����������ķ�Ӧ����֪�Ƽ�������������ʵ�����Ϊ0.1mol��ˮ������Ϊ100g������˵����ȷ���ǣ�������| A�� | �ס��ҡ���������������ԭ��Ӧ | |
| B�� | ��Ӧ�����ӷ���ʽΪNa+2H2O�TNa++2OH-+H2�� | |
| C�� | ����ȫ��Ӧ���ܲ���0.05molO2��ת�Ƶ���0.1mol | |
| D�� | �ס��ҡ�����ַ�Ӧ��������Һ�����������ֱ�Ϊw1��w2��w3����2w1=w2=w3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | MnO2��MnO4- | B�� | A1O2-��Al��OH��3 | C�� | AsO33-��AsO43- | D�� | MnO2��MnCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/L NaOH ��Һ��K+��Na+��SO42-��CO32- | |
| B�� | 0.1mol/L Na2CO3��Һ��K+��Ba2+��NO3-��Cl- | |
| C�� | 0.1mol/L FeCl3��Һ��K+��H+��I-��SCN- | |
| D�� | pH=1����Һ�У�K+��Fe2+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
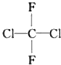 ��
��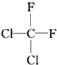 ��
��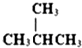 ��
�� ��HCOOCH3��CH3COOH
��HCOOCH3��CH3COOH�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com