
| ʵ����� | Ԥ����������� |
| ����1��������ϡH2SO4����������Ʒ���Թ��У������ܽ⣻ | ��Һ�ʻ���ɫ��˵����Һ�п��ܺ�Fe2+��Fe3+�� |
| ����2��ȡ������Һ���� ��0.01mol/L��KMnO4��Һ�� | ���Ϻ�ɫ����ȥ����˵�������к���Ԫ�صļ�̬+3�ۣ����Ϻ�ɫ��ȥ�� ��˵�������к�����Ԫ�صļ�̬Ϊ+2�� |
| ����3����ȡ������Һ���� ��20%��KSCN��Һ�� | ����Һ��ΪѪ��ɫ����˵�������к���Ԫ�صļ�̬+3�ۣ�����Һ�ޱ仯����˵�������к���Ԫ�صļ�̬+2�ۣ� |
���� ��1��ϡHNO3��ǿ�����ԣ��ɽ�+2����������Ϊ+3�۵�����
��2���ٸ������������������Ĵ��ڼ�̬������������������Ĵ��ڼ�̬��+2��+3�ۣ�
�۸��ݵ�Fe2+��Fe3+���ʼ���������KMnO4��Һ���Ϻ�ɫ��ȥΪ��ɫ��Һ����˵����������Ԫ�صļ�̬��+2�ۣ�ѡ��KSCN��Һ����+3�۵�����
��� �⣺��1��ϡHNO3��ǿ�����ԣ��������ļ�̬Ϊ+2�ۣ�������Ϊ��3��ͬ����ʹKSCN��Һ��Ѫ��ɫ���ʸý��۲���ȷ��
�ʴ�Ϊ����������Fe2+�ᱻ��������ΪFe3+������֪ԭ������һ���Ƿ����Fe3+��
��2�������������������Ĵ��ڼ�̬��+2��+3�ۣ����ԣ���������Ԫ�صļ�̬�������������̬ȫ��Ϊ+3�ۣ���̬ȫ��Ϊ+2�ۣ���̬Ϊ+3�ۡ�+2�ۣ�
�ʴ�Ϊ����������Ԫ�صļ�̬ȫ��Ϊ+2�ۣ���������Ԫ�صļ�̬Ϊ+3�ۡ�+2�ۣ�
�����ʵ�鷽��֤����ļ��裬Ϊ̽����Ԫ�صļ�̬���Ƚ����������������ܽ⣬+2�������л�ԭ�ԣ��ױ�������ѡ��KMnO4��Һ���飬
��KMnO4��Һ���Ϻ�ɫ��ȥΪ��ɫ��Һ����˵����������Ԫ�صļ�̬��+2�ۣ�������ɫ����˵����������Ԫ�صļ�̬����+2�ۣ������1������
Fe3+��KSCN��Һ��Ѫ��ɫ��ѡ��KSCN��Һ����+3�۵��������������KMnO4��Һ���Ϻ�ɫ��ȥΪ��ɫ��Һ������Һ��ΪѪ��ɫ����˵����������Ԫ�صļ�̬��+3�ۣ������3����������Һ�����Ա仯����˵����������Ԫ�صļ�̬����+3�ۣ�ֻ��+2�ۣ������2����
�ʴ�Ϊ��
| ʵ����� | Ԥ����������� |
| 0.01mol/L��KMnO4��Һ��Һ�� | �Ϻ�ɫ����ȥ���Ϻ�ɫ��ȥ�� |
| 20%��KSCN��Һ�� | ��Һ��ΪѪ��ɫ����Һ�ޱ仯 |
���� �����������仯����Ϊ���壬�ۺϿ���ѧ����ʵ�����������������������ͽ�������������ѶȽϴ������������������ʱ仯��ʵ������ǽ���ؼ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ҹ��нϳ��ĺ����ߣ�����ĺ�����һ�����������Դ�������ı��⣮Ŀǰ��������������о���γ�����ú�����Դ��
�ҹ��нϳ��ĺ����ߣ�����ĺ�����һ�����������Դ�������ı��⣮Ŀǰ��������������о���γ�����ú�����Դ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | ���� | ������ | ̼�� | ������ |
| ����ƽ�� ���� | Ka=1.75��10-5 | Ka=2.98��10-8 | Ka1=4.30��10-7 Ka2=5.61��10-11 | Ka1=1.54��10-2 Ka2=1.02��10-7 |
| A�� | 25�棬�����ʵ���Ũ�ȵ�CH3COO-��ClO-��CO32-��SO32-���������������ǿ����ClO- | |
| B�� | ������SO2ͨ��Na2CO3��Һ�з�Ӧ�����ӷ���ʽΪ��SO2+H2O+2CO32-�T2HCO3-+SO32- | |
| C�� | ������SO2ͨ��Ca��ClO��2��Һ�з�Ӧ�����ӷ���ʽΪ��SO2+H2O+Ca2++2ClO-�TCaSO3��+2HClO | |
| D�� | ����CO2ͨ��NaClO��Һ�з�Ӧ�����ӷ���ʽΪ��CO2+H2O+2ClO-�TCO32-+2HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ԭ�Ӱ뾶�Ƚϣ�X��Y��Z��W | |
| B�� | X2H4��H2W����ʹ��ˮ��ɫ | |
| C�� | X���⻯��ķе�һ������Y���⻯��ķе� | |
| D�� | ����������Ԫ���У�Z������������ˮ���������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
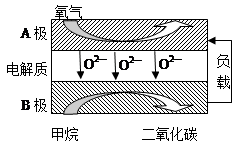
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O��Na2O2 | B�� | Na2O2��Na2CO3 | C�� | Na2CO3 | D�� | Na2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
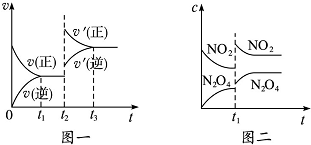 ��֪ͼһ��ʾ���ǿ��淴ӦC��s��+H2O��g��?CO��g��+H2��g����H��0�Ļ�ѧ��Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ��ͼ����ʾ���ǿ��淴ӦN2O4��g��?2NO2��g����H��0��Ũ�ȣ�c����ʱ�䣨t���ı仯���������˵������ȷ���ǣ�������
��֪ͼһ��ʾ���ǿ��淴ӦC��s��+H2O��g��?CO��g��+H2��g����H��0�Ļ�ѧ��Ӧ���ʣ�v����ʱ�䣨t���Ĺ�ϵ��ͼ����ʾ���ǿ��淴ӦN2O4��g��?2NO2��g����H��0��Ũ�ȣ�c����ʱ�䣨t���ı仯���������˵������ȷ���ǣ�������| A�� | ͼһt2ʱ�ı�������������������¶Ȼ�������ѹǿ | |
| B�� | ͼһt2ʱ�ı������������ѹǿ����Ӧ�ġ�H���� | |
| C�� | ͼ��t1ʱ�ı�������������������¶� | |
| D�� | ��ͼ��t1ʱ�ı������������ѹǿ�����������ƽ����Է������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | ���� | ��Һ�� | ������ | |
| A | Pt | Pt | CuCl2 | CuCl2���� |
| B | Pt | Pt | H2SO4 | H2O |
| C | Pt | Pt | NaCl | ���� |
| D | Pt | Pt | CuSO4 | CuO |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com