
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g)2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�

(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
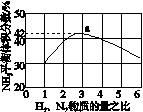
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
(1)2NH4HS��O2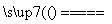 2NH3��H2O��2S��
2NH3��H2O��2S��
(2)a��90%
(3)14.5%
(4)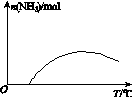
(5)������ԭ������ѹ������Һ����δ��Ӧ��N2��H2ѭ��ʹ��
[����] (1)�������֪Ϊ�����е�O2��������������Ϊ���ʣ����ݵ����غ㽫����ʽ��ƽ���ɡ�(2)��Ӧ��Ϊ�������ʵ�����������ȷ�Ӧ������ѹǿʹƽ�����ƣ�����Ӧ���ʼ�С��d�����������ܸı䷴Ӧ�ȣ������ܸı�H2�İٷֺ�����c��������ˮ����Ũ�����ʹ��Ӧ���������Լ�ƽ�����ƣ�������H2�İٷֺ���ȴ��С��b���������¶ȷ�Ӧ����������ƽ�������ƶ���H2�İٷֺ�������a�ԡ�CO��H2�Ļ��������ˮ�����ķ�Ӧ�У���Ӧ��ϵ�е���������ʵ������䣬��1 molCO��H2�Ļ������μӷ�Ӧ����1.18 mol�������˵����0.18 mol ˮ�����μӷ�Ӧ������ݷ���ʽ�ڿ�֪�μӷ�Ӧ��COҲΪ0.18 mol������ת����Ϊ ��100%��90%��
��100%��90%��
(3)��ͼ�п�����N2��H2���ʵ�����Ϊ1��3ʱ��NH3��ƽ������������Ϊ42%����ƽ��ʱת����N2�����ʵ���Ϊx mol��������ʽ��
����������N2��3H2 2NH3
2NH3
��ʼ(mol): 1 3 0
ת��(mol): x 3x 2x
ƽ��(mol): 1��x 3�� 3x 2x
 ��100%��42%����x��0.59
��100%��42%����x��0.59
��ƽ��ʱN2���������Ϊ ��100%��14.5%��(4)��ͼʱҪע�ʼʱNH3���ʵ����������࣬����Ϊ��Ӧ�������(��Ӧδ��ƽ��)���ﵽһ���̶Ⱥ�Ӧ�ﵽƽ�����ʱ�¶ȼ������ߣ�ƽ�������ƶ���NH3�����ʵ�����С��(5)�Ƚ���������ʹ��Ҫ���ȵ����ʵõ����ȣ�������ʹ��Ҫ��ȴ�����ʵõ���ȴ���ܳ�������������ϳɰ���ӦΪ�������ʵ�����С�ķ�Ӧ����ѹ���ڷ�Ӧ������У����⣬ѭ�����ÿɷ�������ԭ�ϣ����ԭ�������ʡ�
��100%��14.5%��(4)��ͼʱҪע�ʼʱNH3���ʵ����������࣬����Ϊ��Ӧ�������(��Ӧδ��ƽ��)���ﵽһ���̶Ⱥ�Ӧ�ﵽƽ�����ʱ�¶ȼ������ߣ�ƽ�������ƶ���NH3�����ʵ�����С��(5)�Ƚ���������ʹ��Ҫ���ȵ����ʵõ����ȣ�������ʹ��Ҫ��ȴ�����ʵõ���ȴ���ܳ�������������ϳɰ���ӦΪ�������ʵ�����С�ķ�Ӧ����ѹ���ڷ�Ӧ������У����⣬ѭ�����ÿɷ�������ԭ�ϣ����ԭ�������ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
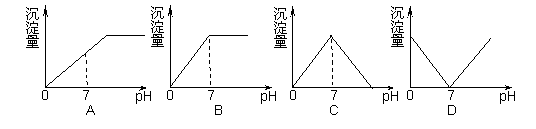
��ʢ��NaHSO4��Һ���ձ��в��ϵ�Ba(OH)2��Һ������Ba(OH)2��Һ�IJ��ϵ��룬��Һ�в����ij�������pH�仯�����ȷ����
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й������ʷ����˵����ȷ����(����)
A�����ʯ���������ڵ���
B��Ư�ۡ�ʯӢ�����ڴ�����
C���Ȼ�李������ᶼ����ǿ�����
D�������ǡ������ʶ����ڸ߷��ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
(1)��ԭ������������˳��(ϡ���������)������˵����ȷ����________��
a��ԭ�Ӱ뾶�����Ӱ뾶����С
b�������Լ������ǽ�������ǿ
c���������Ӧ��ˮ������Լ�����������ǿ
d�����ʵ��۵㽵��
(2)ԭ�������������������������ͬ��Ԫ������Ϊ________�������������ļ���������________��
(3)��֪��
| ������ | MgO | Al2O3 | MgCl2 | AlCl3 |
| ���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
| �۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����__________________________________��
����ʱ�����Al2O3�������AlCl3��ԭ����______________________________��
(4)�����(�۵�1410 ��)�����õİ뵼����ϡ��ɴֹ��ƴ���������£�
Si(��)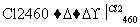 SiCl4
SiCl4 SiCl4(��)
SiCl4(��)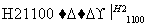 Si(��)
Si(��)
д��SiCl4�ĵ���ʽ��________________����������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(5)P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3 ��b��HI c��SO2 d��CO2
(6)KClO3������ʵ������O2�������Ӵ�����400 ��ʱ�ֽ�ֻ���������Σ�����һ�����������Σ���һ���ε��������Ӹ�����Ϊ1��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
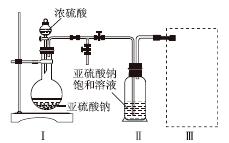
(1)װ�â��в�������Ļ�ѧ����ʽΪ______________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����______��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ________(�����)��
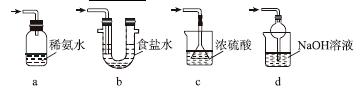
ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO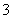 �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH����b������Ba(OH)2��Һ
c���������� d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)���Na2S2O5�����ڿ������ѱ�������ʵ�鷽����
________________________________________________________________________
________________________________________________________________________��
ʵ���������Ѿ��п��������������IJⶨ
(6)���ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2��I2��2H2O===H2SO4��2HI)
�ٰ���������ʵ�飬���ı�I2��Һ25.00 mL���ô�ʵ������Ʒ�п��������IJ�����(������SO2����)Ϊ________g��L��1��
��������ʵ������У����в���HI��������������ⶨ���________(�ƫ�ߡ���ƫ�͡����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������������ϡ���ᷴӦ���ų�NO���ʵ���������(����)
A��FeO B��Fe2O3 C. FeSO4 D��Fe3O4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС����CoCl2��6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����X��Ϊȷ������ɣ���������ʵ�� ��
�ٰ��IJⶨ����ȷ��ȡw g X��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1 mL cl mol��L��1���������Һ���ա�����������ȡ�½���ƿ����c2 mol��L��1NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2 mL NaOH��Һ��
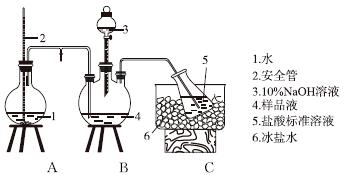
���IJⶨװ��(��ʡ�Լ��Ⱥͼг�װ��)
���ȵIJⶨ��ȷ��ȡ��ƷX�������Һ����AgNO3����Һ�ζ���K2CrO4��ҺΪָʾ���������ֵ���ɫ����������ʧΪ�յ�(Ag2CrO4Ϊש��ɫ)��
�ش��������⣺
(1)װ���а�ȫ�ܵ�����ԭ����__________________________________________��
(2)��NaOH����Һ�ζ���ʣ��HClʱ��Ӧʹ��________ʽ�ζ��ܣ���ʹ�õ�ָʾ��Ϊ________��
(3)��Ʒ�а���������������ʽΪ________��
(4)�ⶨ��ǰӦ�ö�װ�ý��������Լ��飬�������Բ��òⶨ�����________(�ƫ�ߡ���ƫ�͡�)��
(5)�ⶨ�ȵĹ����У�ʹ����ɫ�ζ��ܵ�ԭ����____________________���ζ��յ�ʱ������Һ��c(Ag��)��2.0��10��5 mol��L��1��c(CrO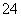 )Ϊ________mol��L��1��[��֪��Ksp(Ag2CrO4)��1.12��10��12]
)Ϊ________mol��L��1��[��֪��Ksp(Ag2CrO4)��1.12��10��12]
(6)���ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3���ܵĻ��ϼ�Ϊ________���Ʊ�X�Ļ�ѧ����ʽΪ______________________________________��X���Ʊ��������¶Ȳ��ܹ��ߵ�ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������µķ�Ӧ��ϵ��
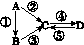
ͼG52
(1)��A�ǵ���ɫ���壬C��D�������C������������Ҫ���ʣ���CҲ����㷺����;��д�����е�2����;��__________________________��
(2)��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�������Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ��
__________________________________��
(3)��D���ʾ������ԣ��ڡ��۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧʱͨ�������һ����������ЧӦ����Ҫ���塣�жϵ���A��Ԫ�������ڱ��е�λ�ã�__________________��
(4)��A��̫���ܵ���õĹ�����ϡ�C��DΪ���Σ������������ơ������Ԫ��Ϊͬһ���壬����Һ���Լ��ԡ�д���ڷ�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
D�Ļ�ѧʽ��______��
(5)��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڡ��ݷ�Ӧ���õ�ͬһ�ַǽ������ʡ�C����Һ����ʴ��ӡˢͭ��·�壬д���÷�Ӧ�����ӷ���ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������OH�������·���ˮ�ⷴӦ��
O2NC6H4COOC2H5��OH�� O2NC6H4COO����C2H5OH
O2NC6H4COO����C2H5OH
���ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050 mol��L��1��15 ��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ���ش��������⣺
| t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
| ��/% | 0 | 33.0 | 41.8 | 48.8 | 58.0 | 69.0 | 70.4 | 71.0 | 71.0 |
(1)��ʽ����÷�Ӧ��120��180 s��180��240 s �����ƽ����Ӧ����________��________���Ƚ����ߴ�С�ɵó��Ľ�����____________________��
(2)��ʽ����15 ��ʱ�÷�Ӧ��ƽ�ⳣ��________��
(3)Ϊ���O2NC6H4COOC2H5��ƽ��ת���ʣ������ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��________(Ҫ��д������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com