
ijͬѧͨ������ʵ���ɹ�ҵ����м�Ʊ�FeSO4��7H2O���壺
�ٽ�5����Na2NO3��Һ���뵽ʢ�з���м���ձ���(��û)�����������Ӻ�Na2CO3��Һ��ȥ��Ȼ����ˮϴ��2��3�飮
����ϴ�ӹ��ķ���м�м��������ϡH2SO4�������¶���50��80��֮������м�ľ���
�۳��ȹ��ˣ�����Һת�뵽�ܱ������о��ã���ȴ�ᾧ��
�ܴ��ᾧ����˳����壬��������ˮϴ�Ӿ���2��3�Σ�������ֽ���������ɣ�
�ݽ��Ƶõľ������һ��С���ƿ�У��ܷⱣ�森
������������⣺
(1)ʵ��ٵ�Ŀ����________�����м��ȵ�ԭ���������________��
(2)ʵ������Բ�������������________��
(3)ʵ�������������ˮϴ�Ӿ��壬��Ŀ���ǣ�________��________��
�����𰸣�(1)����м�����ۡ����ȴ�ʹNa2CO3ˮ��̶�����ʹ��Һ������ǿ[��![]() ��H2O
��H2O![]()
![]() ��OH��ˮ�ⷽ�����ȣ���������ʹƽ����ˮ�ⷽ���ƶ���Һ������ǿ]���ȥ��������
��OH��ˮ�ⷽ�����ȣ���������ʹƽ����ˮ�ⷽ���ƶ���Һ������ǿ]���ȥ��������
����(2)Ӧ����м����(��Ӧ��Ӧ����мʣ��)���������Fe3+����
����(3)��ȥ�������H2SO4�����ʡ�������ˮ���ɽ��;������
����˼·���������⿼�������������Ʊ������ڷ���м��մ�����ۣ�������Ҫ�ü�����Һ����ϴ�ӣ��������ı��������������������ᷴӦ����ܺ��������ӣ�����Ϊ��֤������������Ӧʹ��м������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٽ�5%Na2CO3��Һ���뵽ʢ�з���м���ձ���(��û)�����������Ӻ�Na2CO3��Һ��ȥ��Ȼ����ˮϴ��2��3�顣
����ϴ�ӹ��ķ���м�м��������ϡH2SO4�������¶���50��
�۳��ȹ��ˣ�����Һת�뵽�ܱ������о��ã���ȴ�ᾧ��
�ܴ��ᾧ����˳����壬��������ˮϴ�Ӿ���2��3�Σ�������ֽ���������ɡ�
�ݽ��Ƶõľ������һ��С���ƿ�У��ܷⱣ�档
������������⣺
(1)ʵ��ٵ�Ŀ����____________�����м��ȵ�ԭ���������________________________��
(2)ʵ������Բ�������������_________________________________________________��
(3)ʵ�������������ˮϴ�Ӿ��壬��Ŀ���ǣ�____________��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ��У������ѧ���������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
�̷���FeSO4?7H2O��������ȱ����ƶѪ����Чҩ��ijѧУ�Ļ�ѧ��ȤС���ͬѧ���̷����������µ�̽����
FeSO4?7H2O���Ʊ�
�û�ѧ��ȤС���ͬѧ��ʵ����ͨ������ʵ���ɷ���м������������ͭ�������������ʣ��Ʊ�FeSO4��7H2O���壺
����5%Na2CO3��Һ���뵽ʢ��һ��������м���ձ��У����������ӣ�����������ȥ
Na2CO3��Һ��Ȼ����м��ˮϴ��2��3�顣
����ϴ�ӹ��ķ���м�м��������ϡ���ᣬ�����¶���50��80��֮������м�ľ���
�����ȹ��ˣ�����Һת�뵽�ܱ������У����á���ȴ�ᾧ��
�����ᾧ��Ϻ��˳����壬��������ˮϴ��2��3�Σ�������ֽ���������ɣ�
�����Ƶõ�FeSO4��7H2O�������һ��С���ƿ�У��ܱձ��档
��ش��������⣺
��1��ʵ�鲽������Ŀ��������������������������������
��2��ʵ�鲽�������Բ���������������������������������������
��3��Ϊ��ϴ�ӳ�ȥ������渽�ŵ���������ʣ�ʵ�鲽��������������ˮϴ�Ӿ��壬ԭ������������������������������������������ ��
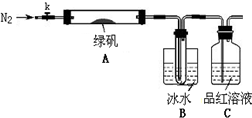
������̽���̷���FeSO4��7H2O���ȷֽ�IJ���
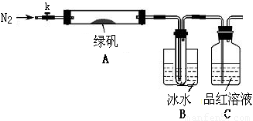 ��֪SO3���۵���16.8��C���е���44.8��C����С���������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
��֪SO3���۵���16.8��C���е���44.8��C����С���������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
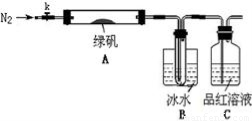
��ʵ����̡�
���������Ӻ��װ��A��B�����ԣ�
��ȡһ�����̷���������A�У�ͨ��N2������װ���ڵĿ������ر�k���þƾ��Ƽ���Ӳ�ʲ����ܣ�
���۲쵽A �й��������ɫ��B���Թ��ռ�����ɫҺ�壬C����Һ��ɫ��
����A�з�Ӧ��ȫ����ȴ�����º�ȡ������Ӧ��������Թ��У����������ܽ⣬ȡ�������뼸��KSCN��Һ����Һ���ɫ��
����Bװ�õ��Թ��е��뼸��BaCl2��Һ����Һ����ǡ�
(4��ʵ��������
����1��B���ռ�����Һ����?????????????????? ��
����2��C����Һ��ɫ������֪��������???? ?????????????? ��
����3���ۺϷ�������ʵ������������֪�������һ����Fe2O3��
��ʵ�鷴˼��
��5����ָ����С����Ƶ�ʵ��װ�õ����Բ��㣺??????????????????????????? ��
��6���ֽ��Ĺ����п��ܺ�������FeO��ȡ����ʵ�����������ܽ�����Һ�������Թ��У�ѡ��һ���Լ����𣬸��Լ�����ʵ���?????????? ��
a����ˮ��KSCN��Һ???? b������KMnO4��Һ????? c��H2O2???? d��NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٽ�5��Na2CO3��Һ���뵽ʢ�з���м���ձ���(��û)�����������Ӻ�Na2CO3��Һ��ȥ��Ȼ����ˮϴ��2��3�顣
����ϴ�ӹ��ķ���м�м��������ϡH2SO4�������¶���50�桪80��֮������м�ľ���
�۳��ȹ��ˣ�����Һת�뵽�ܱ������о��ã���ȴ�ᾧ��
�ܴ��ᾧ����˳����壬��������ˮϴ�Ӿ���2��3�Σ�������ֽ���������ɡ�
�ݽ��Ƶõľ������һ��С���ƿ�У��ܷⱣ�档
��ش��������⣺
(1)ʵ��ٵ�Ŀ����______________�����м��ȵ�ԭ����������_______________________
________________________________________________________________��
(2)ʵ������Բ�������������__________________________________________________��
(3)ʵ�������������ˮϴ�Ӿ��壬��Ŀ���ǣ�______________��______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com