
��ҵ���Ի�ͭ��(��Ҫ�ɷ���CuFeS2�����ʲ�����ˮ����)Ϊԭ�ϣ��Ʊ���ɫ����G���仯ѧʽΪ[Cu(NH3)4]SO4��H2O���漰�������£�
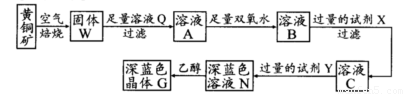
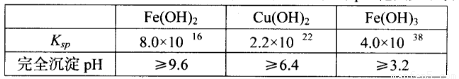
��֪25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ���±���
|
|
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
Ksp |
8.0��10-16 |
2.2��10-22 |
4.0��10-38 |
|
��ȫ����pH |
��9.6 |
��6.4 |
��3.2 |
(1)�ӿ��ͭ�������ʣ��ɲ��õĴ�ʩ�� (д����)��
(2)����˫��ˮ���ܷ�����Ӧ�����ӷ���ʽΪ ��
�Լ�X�Ļ�ѧʽΪ ��
(3)�����£�0.1 mol��L�Լ�Y��pH=11������¶��£��Լ�Y�ĵ��볣��Ϊ ��
��pH��ֽ�����ҺpHֵ�ķ�����
(4)��֪Cu(OH)2+4NH3��H2O [Cu(NH3)4]2++2OH-+4H2O��д���÷�Ӧ��ƽ�ⳣ������ʽ��
��
[Cu(NH3)4]2++2OH-+4H2O��д���÷�Ӧ��ƽ�ⳣ������ʽ��
��
(5)����ҺN�м����Ҵ���Ŀ���� ��
(��14��)
(1)����ͭ����顢�����¶ȡ�����������ٵ�(2��)[��һ���1��]
(2)2Fe2++2H++H2O2=2Fe3++2H2O(2��)[��ƽ�����ѧʽд��0��]
CuO��Cu(OH)2��CuCO3��Cu2(OH)2CO3�е���һ��(2��)
(3)1��10-5 mol/L(2��)
ȡһСƬpH��ֽ���ڲ���Ƭ��������ϣ��ò�������ͷ�ι�ȡ����Һ����pH��ֽ�в�������ɫ�仯�ȶ��������ɫ���Աȣ�����pHֵ��(2��)
(4) 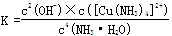 (2��)
(2��)
(5)����G���ܽ�ȣ��ٽ�����ɫ��������(2��)
��������
���������
(1)����ͬ��Ӵ�����������¶ȣ�����������ټ���������Ũ�Ⱦ��ܼӿ컯ѧ��Ӧ���ʡ�
(2)�����ݿ�����ȥ��Ԫ�أ�Ӧ��+2����ת��Ϊ+3����������˫��ˮ�ɴﵽ��Ŀ�ġ�Fe3+ˮ��������Cu2+ǿ��Fe3++3H2O Fe(OH)3+3H+����������ͭ��������ͭ��̼��ͭ���ʽ̼��ͭ���������ɵ��ᣬ��ʹFe3+��ȫˮ�⣬���˳�ȥ������CuO�������������ɡ�
Fe(OH)3+3H+����������ͭ��������ͭ��̼��ͭ���ʽ̼��ͭ���������ɵ��ᣬ��ʹFe3+��ȫˮ�⣬���˳�ȥ������CuO�������������ɡ�
(3)�ɵ��볣������ʽ����ɵá�
(4)��дʱ��������ͭ�����ˮ���������ʽ��
(5)����ɫ�������Ҫ�ɷ���[Cu(NH3)4]SO4��H2O�������Ҵ���Ŀ���ǽ������ܽ�ȣ��ٽ�����������
���㣺�����Թ�������Ϊ�����������˻�ѧʵ�����������������ԭ���ۡ���ѧƽ�����۵����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3?Cu��OH��2 |
| ||
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡȪ���и����ʼ����ۻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ���Ի�ͭ��(��Ҫ�ɷ�CuFeS2)Ϊԭ���Ʊ�����ͭ�����������ֹ��ա�
I.����������:���������Ļ�ͭ�����ʯӢ����ͨ�˿������б��գ������Ƶô�ͭ��
��1�����յ��ܷ�Ӧʽ�ɱ�ʾΪ��2CuFeS2 + 2SiO2+5O2��2Cu+2FeSi03+4SO2�÷�Ӧ����������_____��
��2�����д���SO2�ķ���������������_____
A.�߿��ŷ�
B.�ô�����Һ�����Ʊ���������
C.�ð�ˮ���պ��پ������Ʊ������
D.��BaCl2��Һ�����Ʊ�BaSO3
��3��¯����Ҫ�ɷ���FeO ��Fe2O3 ��SiO2 ,Al2O3�ȣ�Ϊ�õ�Fe2O3�������ܽ��������������,δ�漰���IJ�����_____��
A���ˣ�B�ӹ���NaOH��Һ��C�����ᾧ��D���գ�E��������
II.FeCl3��Һ��ȡ����:��������������ͼ��ʾ

��4�����������У�CuFeS2��FeCl3��Һ��Ӧ�����ӷ���ʽΪ? _____________��
��5���ù��������У�����ѭ�����õ�������_____(�ѧʽ)��
��6������ʯī�缫�����Һ��д�������ĵ缫��ʽ_____________��
��7����ͭ���к�����Pb,����C1һŨ�ȿɿ�����Һ��Pb2+��Ũ�ȣ���c(C1һ)��2mo1��L-1ʱ��Һ��Pb2+���ʵ���Ũ��Ϊ__mol��L-1��[��֪KSP(PbCl2)��1 x 10һ5]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���㽭ʡ�����и����ڶ����¿���ѧ�Ծ��������棩 ���ͣ������
��ҵ���Ի�ͭ��(��Ҫ�ɷ���CuFeS2�����ʲ�����ˮ����)Ϊԭ�ϣ��Ʊ���ɫ����G���仯ѧʽΪ[Cu(NH3)4]SO4��H2O���漰�������£�
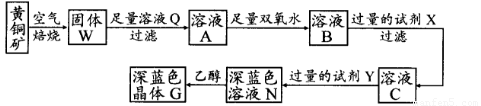
��֪25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ���±���
��1����ͭ���ڿ����б�������������ͭ�ĵͼ����д���䷴Ӧ�Ļ�ѧ����ʽ��???????????????????? ��
��2���Լ�X�Ļ�ѧʽΪ??????????????? ��
��3�������£�0.1 mol��L�Լ�Y��pH=11������¶��£��Լ�Y�ĵ��볣��Ϊ?????????????????? ����pH��ֽ�����ҺpHֵ�ķ�����?????????????????????????????????????????????????? ��
��4������ҺN�м����Ҵ���Ŀ����???????????????????????????????????????????????????? ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com