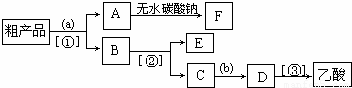
ʵ���Һϳ���������ʱ���õ����������ֲ�Ʒ����ش��������⣺
����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
��1������ƿ�г��˼���
�Ҵ�
�Ҵ�
��
Ũ����
Ũ����
��
����
����
�⣬��Ӧ���뼸�����Ƭ����Ŀ���Ƿ�ֹ��ƿ��Һ�屩�У�
��2������ƿ�м���һ���������Ҵ���Ũ����Ļ��Һ�ķ����ǣ�
������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
������ƿ�м���һ�������Ҵ���Ȼ��������Ũ���������ƿ���ӱ���
��
��3���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫת��Ϊ�������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬ʱ��������ţ�
�ڢܢ�
�ڢܢ�
��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
������Ӧ���������淴Ӧ��������� �ݻ�����и����ʵ�Ũ�Ȳ��ٱ仯
��4��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����

EΪ
�Ҵ�
�Ҵ�
�������ƣ����Լ�a��
����̼������Һ
����̼������Һ
���Լ�bΪ
Ũ����
Ũ����
�����뷽������
��Һ
��Һ
�����뷽������
����
����
��
��5��д���ϳ�����������Ӧ�Ļ�ѧ����ʽ
CH
3COOH+C
2H
5OH
CH
3COOC
2H
5+H
2O
CH
3COOH+C
2H
5OH
CH
3COOC
2H
5+H
2O
��

 ʵ���Һϳ���������ʱ���õ����������ֲ�Ʒ����ش��������⣺
ʵ���Һϳ���������ʱ���õ����������ֲ�Ʒ����ش��������⣺


 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�
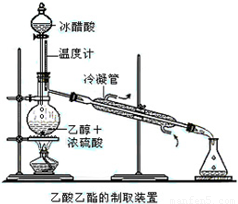
 ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩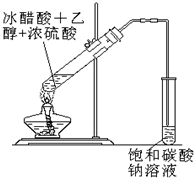 ʵ���Һϳ����������ֲ�Ʒ�ļ���װ�����ң��ɸ�ʵ����Եõ����������ֲ�Ʒ���ݴ���գ�
ʵ���Һϳ����������ֲ�Ʒ�ļ���װ�����ң��ɸ�ʵ����Եõ����������ֲ�Ʒ���ݴ���գ�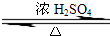 CH3COOCH2CH3+H2O��������Ӧ
CH3COOCH2CH3+H2O��������Ӧ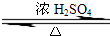 CH3COOCH2CH3+H2O��������Ӧ
CH3COOCH2CH3+H2O��������Ӧ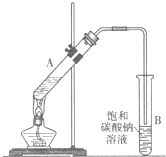 ������������Ҫ�Ļ���ԭ�ϣ�ʵ���Һϳ�����������װ����ͼ��ʾ��
������������Ҫ�Ļ���ԭ�ϣ�ʵ���Һϳ�����������װ����ͼ��ʾ��