
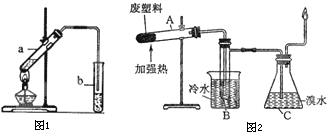
��14�֣���ͼ��ʾΪ�������ȡ����Ӧ��ʵ��Ľ����װ��ͼ������AΪ����֧�ܵ��Թܸ��Ƴɵķ�Ӧ�����������¶˿���һ��С�ף�����ʯ���ޣ��ټ���������м�ۡ�
��д���пհף�
��1����Ӧ����A����μ�����ͱ��Ļ��Һ���������ھͻᷢ����Ӧ���÷�Ӧ����м�۵�������___________________________��д��A�з�����Ӧ�Ļ�ѧ����ʽ���л���д�ṹ��ʽ��________________________________________��
��2���Թ�C�б��������� ����Ӧ��ʼ�۲�D��E��֧�Թܣ�����������Ϊ___________________________________________��
��3��F�з�����Ӧ�����ӷ���ʽ�� ___________________��
��4������������װ���У���ȡ�˷�������ʩ��װ����________________������ĸ���ţ���
��5��ʵ���ҵõ����屽��Ҫ�����²������ƣ������� ��ˮϴ ���ø�������� ��10% NaOH��Һϴ ��ˮϴ ��ȷ�IJ���˳���� ����д��ţ�
A���٢ڢۢܢ� B���ڢܢݢۢ�
C���ܢڢۢ٢� D���ڢܢ٢ݢ�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ʵ������ |
| I������Ϊ�٢ڢ۵�3֧�Թ��У��ֱ����1mL 20%��������Һ�����Թܢں͢��м���0.5mLϡ���ᣬ������3֧�Թ�ͬʱˮԡ����Լ5min | ������������ |
| II��ȡ�Թܢٺ͢ڣ���������������ͭ����Һ������������ | ������������ |
| III��ȡ�Թܢۣ��ȼ���NaOH��Һ����ҺpH�����ԣ��ټ�������������ͭ����Һ������������ | |
| ���ۣ�֤��������ϡ���������·�����ˮ�ⷴӦ | |
| ���� | ���� | ���� | ��ϩ | ��ϩ | �� | �ױ� | ̼ |
| ����������%�� | 12 | 24 | 12 | 16 | 20 | 10 | 6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���������ֻ�ѧ�ս̰� �ս̰� ���ͣ�022
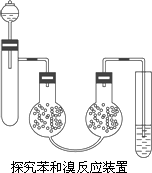
ijѧ��Ϊ̽�������巴Ӧ��������ͼ��ʾװ�ã���һ֧�Թ��м�����˿�ѱ�������4��1(�����)��ϣ��ڷ�Һ©�������3��4 mL���Һ��˫�����չ���ע��һ������Һ�壬����ͨ��ʢ��AgNO3��Һ���Թ��������Һ©����������μ��뱽����Ļ��Һ���۲�����Ӧ��ϣ�ȡ��©��������Ӧ��Ļ��Һע��3 mol��L��1��NaOH��Һ�У���ְ�����۲�����
�����������⣺
(1)������Ļ��Һ����֮��ʼû��������һ�����ӦҺ�ʷ���״������װ�ó����˳Ⱥ�ɫ����������ν�����������
(2)���ܵ�������ʲô��Ϊʲô����ĩ�˲�����Һ�����£����Ҫ�����ܲ���Һ�����£���ĸĽ��취��ʲô��
(3)˫�����չ���ע���Һ����ʲô��������ʲô��
(4)��ƿ�ڵ�����˵���������ַ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
������I��II��С�⡣
������a��e����ѧ��ѧʵ���г����ļ��ֶ���������
��a����Ͳ ��b������ƿ ��c���ζ��� ��d��������ƽ ��e���¶ȼ�
��1�����б�ʾ������ʹ���¶ȵ���__________����д��ţ���
��2���ܹ����Ծ�ȷ��ȡҺ���������_______����д��ţ���
��3�����ڲ�������ʹ�õ������ݱ���ȷ����ƫС����________����д��ţ���
A������Ͳ��ȡһ����Һ��ʱ������Һ�����
B���к͵ζ����յ�ʱ���ӵζ�����Һ�����
C��ʹ������ƿ������Һʱ������Һ�涨��������Һ��Ũ��
��4����ȡ10.5 g������Ʒ��1 g����ʹ�����룩ʱ������Ʒ��������ƽ�����̣���������Ʒ��ʵ������Ϊ_________g��
��5��������ʵ�飺
�ٱ������ȡ����Ӧ �ڱ���������Ӧ
����ȩ��������Ӧ �ܲⶨһ���¶���KNO3���ܽ��
��������������ȡ ��ʯ�ͷ���ʵ��
������Ҫʹ���¶ȼƵ���___________����д��ţ�
��ij��θҩƬ�������Ϊ̼��ƣ�����������CaCO3�����IJⶨ���£�
��������0.1 mol/L��HCl��0.1 mol/L��NaOH��Һ��
��ÿ��ȡһ����ҩƬ��������ͬ��0.2 g�Ĵ�θҩƬ��ĥ������20.00 mL����ˮ��
���Է�̪Ϊָʾ������0.1 mol/L��NaOH��Һ�ζ�������ȥV mL��ζ��յ㣻
�ܼ���25.00 mL 0.1 mol/L��HCl��Һ��
��1��д��ʵ�鲽�裨д���˳��___________________________��
��2������ͼ��ʾ������������0.1 mol/L��HCl��Һ��0.1 mol/L��NaOH��Һ�϶�����Ҫ�������ǣ�����ţ�________������������Һ����Ҫ�IJ��������ǣ����������ƣ�_________________________��

��3������������ҺӦѡ�õ�����ƿ�����_________������ĸ����
A��50 mL 50 mL B��100 mL 100 mL
C��100 mL 150 mL D��250 mL 250 mL
��4��д���йصĻ�ѧ����ʽ______________��
��5��ÿƬθҩ�к�̼��Ƶ�������____________________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��F��G�����л���������ǵĹ�ϵ����ͼ��ʾ��
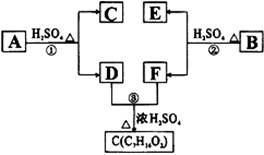
��1��������C�ķ���ʽ��C7H8O��C��FeCl3��Һ����ɫ��C�����ϵ���ԭ�ӱ���ԭ��ȡ
�������ɵ�һ�����ֻ�����֣���C�Ľṹ��ʽΪ
��2��DΪһֱ�����������Է��������Ȼ�����C��С20�����ܸ�NaHCO3��Ӧ�ų�CO2����D����ʽΪ����������������D���еĹ�����������������������������
��3����Ӧ�ٵĻ�ѧ����ʽ������������������������������������������������
��4�����㻯����B����A������ͬ�����ŵ�A��ͬ���칹�壬ͨ����Ӧ�ڻ�����B������E��F��F���ܵĽṹ��ʽΪ������������������������������������
��5��E���ܵĽṹ��ʽ��������������������������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com